PIOMBINO DESE, Italy / Mar 16, 2023 / Business Wire / Stevanato Group S.p.A. (NYSE: STVN) a leading global provider of drug containment, drug delivery, and diagnostic solutions to the pharmaceutical, biotechnology, and life sciences industries, today announced a collaboration with leading Contract Development and Manufacturing Organisation (CDMO), Recipharm. Under the agreement, Stevanato Group will lend its 70+ years of manufacturing experience to support the development and production of pre-fillable syringes for use in Recipharm’s soft mist inhalers. As part of this collaboration, Stevanato Group will provide and manufacture its glass pre-fillable syringe Alba® assembled with the Integrated Spray Module (ISMTM) of Recipharm’s soft mist inhaler technology, the Pre-Filled Syringe Inhaler (PFSITM).
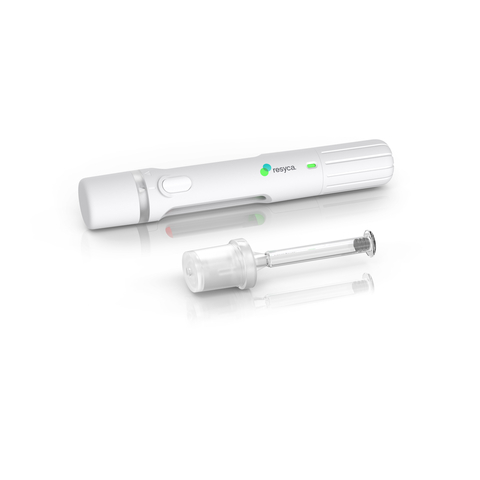
This collaboration highlights Stevanato Group’s capabilities as a one-stop shop offering contract drug manufacturing organizations support in their drug development programs, from the clinical phase through to market release. Stevanato Group’s Alba® syringes feature an internal coating based on silicone oil, which is cross-linked with the glass surface, thereby minimizing sub-visible particle release and aiming to ensure superior performance during delivery.
The combination of the Alba® syringe and Recipharm's innovative soft mist inhaler technology delivers sensitive drug products more efficiently to the respiratory airways and provides biopharma companies with a containment solution featuring enhanced stability and safety. With Stevanato Group taking responsibility for the complete primary drug package, Recipharm’s focus is on the design and manufacture of the spray technology, the inhalation device, and the fill and finish of the product.
Recipharm’s soft mist inhaler (PFSI™) is developed, manufactured, and licensed by Resyca® and is supplied as a pre-filled and ready-to-use inhalation device.
Bernhard Muellinger, General Manager and Chief Operational Officer at Resyca® (a joint venture between Recipharm and Medspray), said: “By leveraging Stevanato Group's integrated capabilities, from plastic injection molding and assembly capabilities to comprehensive scientific and analytical support services, we will be able to offer a turnkey solution, and accelerate and de-risk our pharmaceutical customers’ development programs. This is particularly true for novel inhaled biological products.”
“Biopharma companies are advancing patient care with new, innovative treatments, particularly in biologics and mRNA therapies. These products require specialized, high-performance drug containment systems, like our Alba® syringe platform, together with patient-centric drug delivery devices like the PFSI™. Thanks to our integrated end-to-end capabilities we are able to support our customers at scale with a comprehensive system solution,” said Mauro Stocchi, CBO of Stevanato Group.
Franco Moro, CEO of Stevanato Group, noted: “Creating a network of strategic collaboration partners is an important element of our long-term strategy to match customers’ needs and address self-administration trends in patient care with user-friendly drug delivery devices that provide variable and accurate dosing. This agreement marks another key step in broadening our high-value solutions and integrated capabilities as we continue to diversify and enhance our presence in the drug delivery market of pen injectors, auto-injectors, inhalers, and wearable pods.”
Forward-Looking Statements
This press release may include forward-looking statements. The words "aims", “will”, "aiming", “accelerate”, “are able”, “creating”, “continue”, and similar expressions (or their negative) identify certain of these forward-looking statements. These forward-looking statements are statements regarding the Company's intentions, beliefs or current expectations concerning, among other things, the investments the Company expects to receive, the expansion of manufacturing capacity, the Company’s plans regarding its presence in the U.S. market, business strategies, the Company’s capacity to meet future market demands and support preparedness for future public health emergencies, and results of operations. The forward-looking statements in this press release are based on numerous assumptions regarding the Company’s present and future business strategies and the environment in which the Company will operate in the future. Forward-looking statements involve inherent known and unknown risks, uncertainties and contingencies because they relate to events and depend on circumstances that may or may not occur in the future and may cause the actual results, performance or achievements of the Company to be materially different from those expressed or implied by such forward-looking statements. Many of these risks and uncertainties relate to factors that are beyond the Company's ability to control or estimate precisely, such as future market conditions, currency fluctuations, the behavior of other market participants, the actions of regulators and other factors such as the Company's ability to continue to obtain financing to meet its liquidity needs, changes in the political, social and regulatory framework in which the Company operates or in economic or technological trends or conditions. Readers should therefore not place undue reliance on these statements, particularly not in connection with any contract or investment decision. Except as required by law, the Company assumes no obligation to update any such forward-looking statements.
About Stevanato Group
Founded in 1949, Stevanato Group is a leading global provider of drug containment, drug delivery and diagnostic solutions to the pharmaceutical, biotechnology and life sciences industries. The Group delivers an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle at each of the development, clinical and commercial stages. Stevanato Group’s core capabilities in scientific research and development, its commitment to technical innovation and its engineering excellence are central to its ability to offer value added solutions to clients. To learn more, visit: www.stevanatogroup.com.
About Recipharm
Recipharm is a leading Contract Development and Manufacturing Organisation (CDMO) in the pharmaceutical industry employing almost 9,000 employees. Recipharm offers manufacturing services of pharmaceuticals in various dosage forms, production of clinical trial material and APIs, pharmaceutical product development and development and manufacturing of medical devices. Recipharm manufactures several hundred different products to customers ranging from big pharma to smaller research and development companies. The company operates development and manufacturing facilities in France, Germany, India, Israel, Italy, Portugal, Spain, Sweden, the UK and the US and is headquartered in Stockholm, Sweden.
For more information on Recipharm and our services, please visit www.recipharm.com
About Resyca®
Resyca® was founded in 2020 and is a joint venture between Recipharm and Medspray. Resyca® is part of the Recipharm Advanced Delivery Systems business unit. Resyca® develops and manufactures pocket size, user friendly soft-mist inhalation devices, based on standard pre-filled syringe and filled on standard filling lines. As a soft mist development center with Recipharm, Resyca® offers an end-to-end service for soft-mist inhalation products, ranging from early-stage development to commercial manufacture, including filling, labeling, and packaging.
| Last Trade: | US$20.56 |
| Daily Change: | -0.78 -3.66 |
| Daily Volume: | 610,216 |
| Market Cap: | US$1.020B |
November 11, 2024 November 05, 2024 October 01, 2024 August 29, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB