ENGLEWOOD, Colo. / Aug 14, 2024 / Business Wire / Paragon 28, Inc. (NYSE: FNA), is proud to announce the launch of the SMART28℠ Case Management Portal, a cutting-edge platform that leverages AI and a seamless user experience to coordinate patient-specific surgical plans. It is the first major launch of Paragon 28’s SMART28℠ ecosystem, a portfolio of solutions and products that drive Paragon 28’s SMART28℠ initiative: to improve all aspects of foot and ankle treatments through the utilization of artificial intelligence, data analytics, patient specific algorithms, 3D modeling, and other enabling technologies.
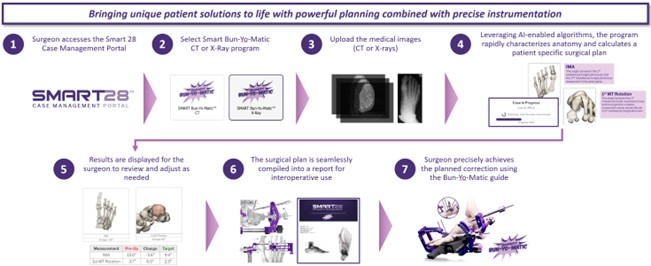
The SMART28℠ Case Management Portal gives surgeons the ability to plan cases, coordinate surgery dates, and submit patient imaging. It will also serve as a direct line of communication with the Paragon 28 engineering and sales teams for real-time support, status updates, and surgical planning insights. The first module to launch within the Case Management Portal is SMART Bun-Yo-Matic℠ for bunion correction.
SMART Bun-Yo-Matic℠ has imaging optionality to input either patient weight-bearing X-rays or weight-bearing CT (“WBCT”) scans. It is the first foot and ankle orthopedic device on the market that allows the user to see the foot as 3D anatomy from two X-ray images. If a surgeon uses WBCT, they have access to “see inside the joint,” allowing the user to assess the joint distance distribution specific to their patient and compare it to normal joint alignment.
SMART Bun-Yo-Matic℠ revolutionizes preoperative planning and correction for hallux valgus by creating plans tailored to the individual patient. AI-driven algorithms and statistical shape modeling integrate with surgeon input, and within 10 minutes, export a surgical plan for correction, notifying the user if abnormal anatomy is detected. SMART BYM℠ allows the surgeon to adjust the plan in real time and exports a new surgical plan incorporating their recommended changes, if desired. The final surgical plan provides the surgeon user specific reference points that allow for additional reproducibility when paired with The Bun-Yo-Matic Lapidus Clamp, which can be used to identify, measure, and hold the planned corrections in place during the procedure.
Dr. Cesar De Cesar Netto, surgeon designer and one of the first users of the SMART Bun-Yo-Matic℠, says of the portal: “The ability to 3D plan your Hallux Valgus correction is crucial for understanding this complex deformity and optimizing multiplanar correction. Even more revolutionary is the capability to apply, control, and achieve the planned correction with the use of the Clamp. Precision down to the millimeter matters, the SMART Bun-Yo-Matic℠ system represents a significant advancement in improving precision and outcomes in Hallux Valgus surgery.”
Paragon 28’s CEO, Albert DaCosta, commented, “We could not be more excited about the launch of our SMART 28 Case Management Portal and SMART Bun-Yo-Matic cleared for weight-bearing CT and X-ray. This marks a tremendous step forward for Paragon 28 and we expect it will make a big impact on the future of foot and ankle surgery. With the SMART Bun-Yo-Matic℠, surgeons are able to efficiently plan a procedure in 3D and have the ability to precisely implement the plan with full intraoperative control.”
The SMART28℠ Case Management Portal is a foundational tool for future modules to span many additional foot and ankle conditions. With the introduction of SMART Bun-Yo-Matic℠, Paragon 28 has taken a giant leap forward in paving the way for surgeons to utilize artificial intelligence to optimize surgical outcomes for their patients.
For more information regarding the SMART 28℠ ecosystem and modules please visit: https://smart.paragon28.com/
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 and its Quarterly Reports on Form 10-Q, as updated periodically with its other lings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could di er from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Cesar De Cesar Netto may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
| Last Trade: | US$10.03 |
| Daily Change: | 0.23 2.35 |
| Daily Volume: | 1,278,132 |
| Market Cap: | US$839.710M |
December 11, 2024 November 12, 2024 October 16, 2024 September 10, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB