MARLBOROUGH, Mass., Oct. 18, 2024 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) today announced it has received U.S. Food and Drug Administration (FDA) approval for the navigation-enabled FARAWAVE™ NAV Ablation Catheter for the treatment of paroxysmal atrial fibrillation (AF) and FDA 510(k) clearance for the new FARAVIEW™ Software, which will combine to provide visualization for cardiac ablation procedures with the FARAPULSE™ Pulsed Field Ablation (PFA) System. These technologies are compatible exclusively with Boston Scientific's existing cardiac mapping technology and the company's latest offering, the OPAL HDx™ Mapping System.
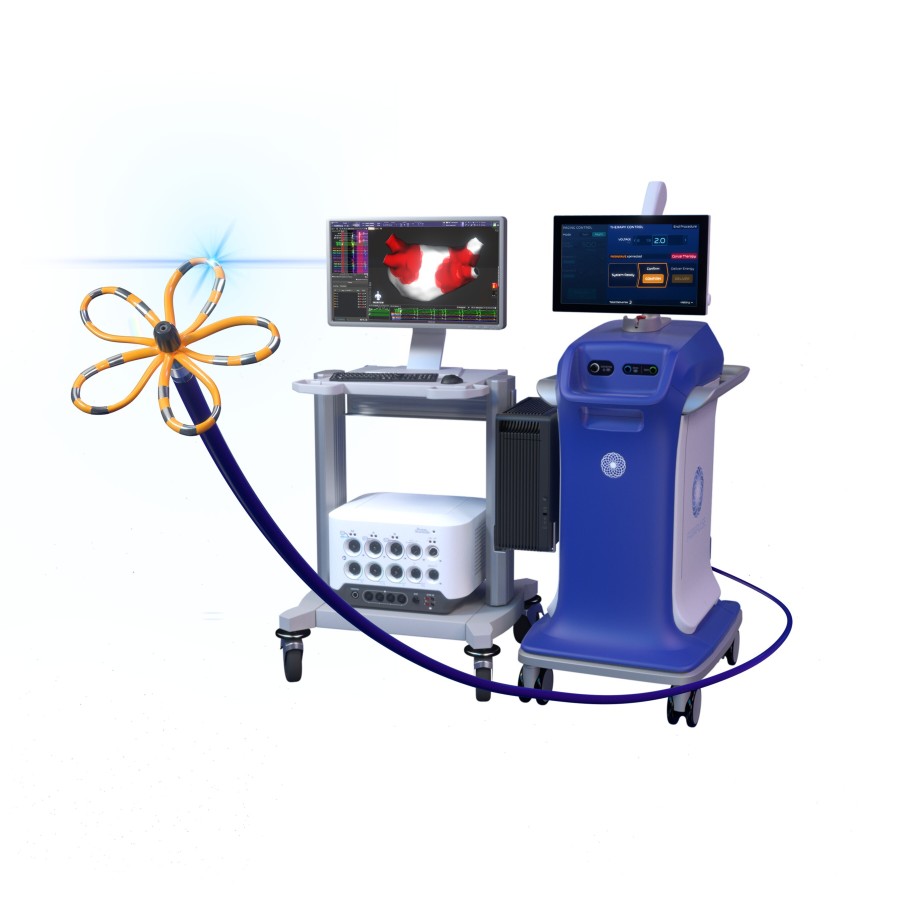
Today, physicians may utilize a separate mapping catheter prior to a cardiac PFA procedure to examine and analyze the heart's electrical patterns and plan the therapeutic applications for each patient. The FARAWAVE NAV Ablation Catheter builds upon the current FARAWAVE catheter by adding magnetic navigation capabilities, enabling cardiac mapping and PFA therapy delivery within a single integrated catheter, while minimizing the need for additional device exchanges. Mapped procedures with the FARAPULSE PFA System are visually depicted for physicians via the FARAVIEW Software, which offers a dynamic view of catheter placement, shape and rotation.
"The addition of the FARAWAVE NAV Ablation Catheter and FARAVIEW Software to our portfolio provides a next-level pulsed field ablation mapping experience with cost-effective tools developed specifically and uniquely for the FARAPULSE PFA System," said Nick Spadea-Anello, president, Electrophysiology, Boston Scientific. "Built on this safe and clinically-proven platform – which has been used to treat more than 125,000 patients globally – the visualization capabilities offered by these new technologies are designed to increase confidence in therapy delivery and simplify mapped workflows during PFA procedures."
The latest advancement within the Boston Scientific PFA portfolio, the FARAVIEW Software magnetically tracks the FARAWAVE NAV Ablation Catheter, which allows physicians to see where pulsed fields have been applied and visualize cumulative therapy delivery to guide ablation strategy. Delivery of PFA with the FARAWAVE NAV Ablation Catheter can be tracked through automated tagging technology, which is intended to assist physicians in planning, executing and confirming application of therapy by showing the approximate pulsed field locations within the heart, based on the catheter's position.
"In clinical use, the FARAVIEW Software and FARAWAVE NAV Ablation Catheter produced detailed cardiac maps that could improve guidance, limit fluoroscopy times and assist physicians in assessing the location of energy delivered during PFA procedures," said Vivek Reddy, M.D., director of electrophysiology, Mount Sinai Fuster Heart Hospital, New York. "The addition of navigation and visualization capabilities to the FARAPULSE PFA System could aid workflow efficiency and enhance the treatment physicians can provide to patients living with AF."
Boston Scientific will immediately launch the FARAWAVE NAV Ablation Catheter and FARAVIEW Software in the U.S. More information is available here.
*Dr. Vivek Reddy is a paid consultant of Boston Scientific Corporation. He has not been compensated in connection with this press release.
About Boston Scientific
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 45 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions. Learn more at www.bostonscientific.com and connect on LinkedIn and X, formerly Twitter.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact, and new and anticipated product approvals and launches. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; manufacturing, distribution and supply chain disruptions and cost increases; variations in outcomes of ongoing and future clinical trials and market studies; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements, except as required by law. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Steve Bailey
Media Relations
(651) 582-4343 (office)
This email address is being protected from spambots. You need JavaScript enabled to view it.
Jon Monson
Investor Relations
(508) 683-5450
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$89.68 |
| Daily Change: | -0.98 -1.08 |
| Daily Volume: | 2,872,616 |
| Market Cap: | US$131.830B |
November 25, 2024 November 15, 2024 November 04, 2024 October 30, 2024 | |

Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB