FRANKLIN LAKES, N.J., April 11, 2023 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today launched a new, easy-to-use advanced ultrasound device with a specialized probe designed to provide clinicians with optimal IV placement.
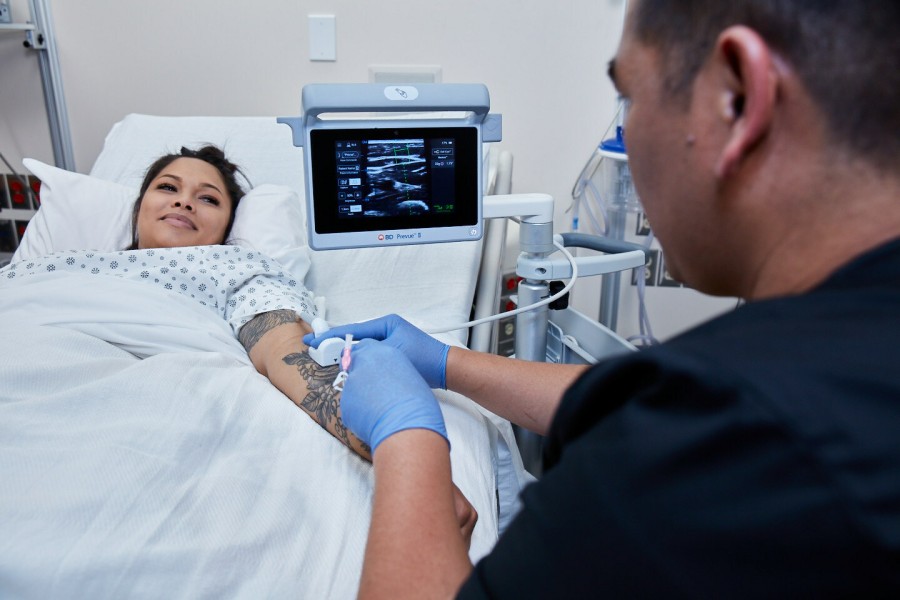
The BD Prevue™ II System addresses an unmet need in IV access through real-time needle depth markers. The system features the BD Cue™ Needle Tracking System, offering a high-quality ultrasound image of the needle trajectory, and is compatible with BD Cue™ Needle Tracking-enabled catheters. Simulated studies show that pairing a needle-tracking system with ultrasound guidance may help reduce the number of attempts and time to successful vessel access -- which, according to the American Institute of Ultrasound in Medicine, may make vascular access procedures safer and easier for clinicians and patients.1
More than 90 percent of hospitalized patients receive IV therapy through a peripheral IV catheter2 -- representing hundreds of millions of patients a year.3 However, nearly two-thirds4 of these patients have vessels that are difficult to visualize and access, often resulting in additional needlesticks, vessel damage and infiltration into the surrounding tissue.
"Difficult IV access remains far too common today, but the availability of the BD Prevue™ II System supports a future where every needlestick has a predefined pathway for successful placement," said Eric Borin, worldwide president of Medication Delivery Solutions at BD. "BD is advancing the vision of a 'One-Stick Hospital Stay,' and first-stick success is the first requirement to fulfill that vision. First-stick success also reduces the pain and anxiety patients often experience while undergoing multiple IV access attempts and provides clinicians with IV workflow efficiencies and confidence."
The system is designed with clinician-friendly features to help expand the use of ultrasound in IV placement. For example, the BD Prevue™ II System offers a specialized probe designed to help reduce the learning curve — ensuring that clinicians can use the technology without changing their current insertion technique or field of view.
"Patients living with chronic conditions like heart failure may suffer from poor circulation and large amounts of scar tissue from frequent venous access making their veins more difficult to locate and creating a challenge for placing a good, lasting peripheral IV line," said Lisa Wilson, registered nurse and night shift lead for the progressive cardiac care unit at INTEGRIS Health Baptist Medical Center in Oklahoma City, Okla. "With the use of ultrasound guidance technology, we are able to quickly and successfully place IVs in our hard-to-stick patients, leading to quicker treatment and fewer sticks for our patients and a better experience for everyone involved."
As the global leader in vascular access solutions, BD is committed to advancing the standard of care for IV therapy and blood draws for patients and health care providers. The availability of the BD Prevue™ II System further drives the BD "One-Stick Hospital Stay" vision to help reduce unnecessary needlesticks by choosing the right vascular access device and placing it successfully the first time. This is one of three pillars of the vision that also includes using one IV line as a single access point for required therapies and blood draws, and optimal maintenance of the IV line to help reduce the risk of complications so it does not have to be replaced and lasts throughout a patient's hospital stay.
1 Alsbrooks, K, Hoerauf, K. Comparative Effectiveness, Efficiency, and ED Nurse Preference Between Two Methods of Visualization for Midline Catheter Insertion: A Pilot Study. SAGE Open Nursing. 2023. |
2 Bahl, A, Hijazi, M, Chen, N, et al. Ultralong versus standard long peripheral intravenous catheters: A randomized controlled trail of ultrasonographically guided catheter survival. Anals of Emergency Medicine. 2020;76(2):134-142 |
3 iData Research Inc. US Market Report Suite for Vascular Access Devices and Accessories. 2020. |
4 Armenteros-Yeguas, V, Garate-Echenique, L, Tomas-Lopez,MA, et al. Prevalence of difficult venous access and associated risk factors in highly complex hospitalized patients. Journal of Clinical Nursing. 2017;26:4267-4275 |
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 77,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media: | Investors: |
Alyssa Kretlow | Francesca DeMartino |
Associate Director, Communications | SVP, Head of Investor Relations |
858.617.2361 | 201.847.5743 |
This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. |

| Last Trade: | US$225.15 |
| Daily Change: | -2.02 -0.89 |
| Daily Volume: | 1,682,996 |
| Market Cap: | US$65.080B |
November 07, 2024 October 30, 2024 October 29, 2024 October 14, 2024 September 26, 2024 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB