FRANKLIN LAKES, N.J., Nov. 2, 2023 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today launched new needle-free blood draw technology compatible with integrated catheters, helping to further enable the company's vision of a "One-Stick Hospital Stay."
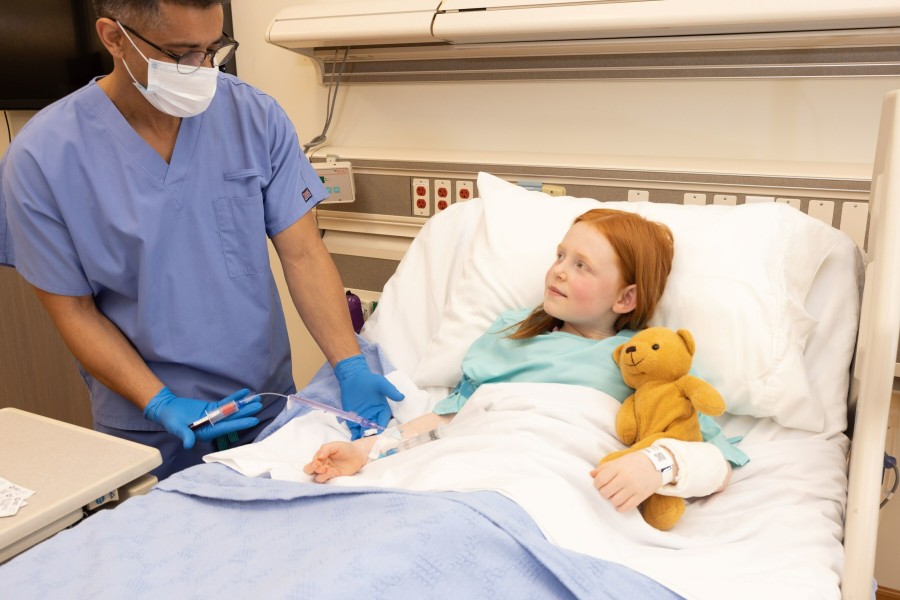
With 510(k) clearance from the U.S. Food and Drug Administration (FDA), the new PIVO™ Pro Needle-free Blood Collection Device features design improvements to achieve the first and only compatibility with integrated and long peripheral IV catheters, including the new Nexiva™ Closed IV Catheter System with NearPort™ IV Access. This expands on current PIVO™ compatibility with traditional short peripheral IV catheters available since 2017.
The new solution combines the clinical benefits shown for the integrated closed Nexiva™ Catheter System, including longer dwell times* and reduced catheter complications, with the ability to draw high-quality blood samples directly from a patient's peripheral IV line with PIVO™ Pro, reducing the need for additional needlesticks.1,2 With needle phobia experienced by more than 60 percent of the adult population, this solution helps improve the patient experience by alleviating fear and anxiety associated with repetitive needlesticks.3
"The latest innovation to our PIVO™ portfolio helps expand needle-free blood collection to even more patients and clinicians as we continue to redefine the standard of care through our 'One-Stick Hospital Stay' vision," said Eric Borin, worldwide president of Medication Delivery Solutions at BD. "This new solution helps to reduce unnecessary and repeat needlesticks in the hospital while elevating clinical outcomes, improving workflow and creating a better experience for clinicians and patients."
Peripheral IV insertion and blood collection are two of the most common in-patient procedures in hospitals touching almost every patient daily.4,5 These invasive procedures are associated with a variety of complications such as poor first-stick insertion success, frequent catheter failures and poor sample quality that can extend hospital stays, increase cost and create a dissatisfying experience.6,7
PIVO™ Pro combined with Nexiva™ with NearPort™ IV Access is designed to access optimal blood draw conditions and help improve clinician efficiency and patient experience.2,8 The innovative line draw solution has been shown to help optimize IV performance, reduce sample errors that can result in redraws and delays in patient care while reducing complications that lead to unnecessary procedures and IV replacements.**,1,2,7 By removing the needle from blood draws and reducing IV replacements, it may reduce the risk of needlestick injury and blood exposure for clinicians while preserving a patient's vessel health.7,9,10
As the global leader in vascular access solutions, BD is committed to advancing the standard of care for IV therapy and blood draws for patients and health care providers. The latest innovation in the BD Peripheral Line Draw Solution further drives the BD "One-Stick Hospital Stay" vision across each of its three pillars ― including (1) helping to reduce unnecessary needlesticks by choosing the right vascular access device and placing it successfully the first time; (2) using one IV line as a single access point for required therapies and blood draws; and (3) optimal maintenance of the IV line to help reduce the risk of complications so it does not have to be replaced and lasts throughout a patient's hospital stay.
*Compared to open catheter system
**Clinical studies were done on previous generations of the PIVO™ Blood Collection Device and Nexiva™ Catheter System. PIVO™ Pro and Nexiva™ with NearPort IV Access are the next generations of their respective product families.
About PIVO™
Since its introduction in 2017, the PIVO™ technology has been advancing the standard of care by minimizing the number of needlesticks for patients by using an existing peripheral IV catheter line to collect a high-quality, reliable blood sample through a singular access point. To date, more than 3.5 million PIVO™ procedures have been completed in the U.S. – each of these representing one or more needlesticks eliminated for patients. For more information about the BD Peripheral Line Draw Solution, including PIVO™ Pro and Nexiva™ with NearPort™ IV Access, visit https://www.bd.com/PIVO.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 77,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media: | Investors: |
Alyssa Kretlow | Greg Rodetis |
BD Corporate Communications | BD Investor Relations |
551.238.4391 | 201.847.6875 |
This email address is being protected from spambots. You need JavaScript enabled to view it. | This email address is being protected from spambots. You need JavaScript enabled to view it. |
References:
1 González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
2 Pendleton B, LaFaye R. Multicenter study of needle-free blood collection system for reducing specimen error and intravenous catheter replacement. J Healthc Qual. 2022;44(2):e24-e30. doi:10.1097/JHQ.0000000000000331.
3 Alsbrooks K, Hoerauf K. Prevalence, causes, impacts, and management of needle phobia: An international survey of a general adult population. Concerto C, ed. PLOS ONE. 2022;17(11):e0276814. http://doi.org/10.1371/journal.pone.0276814.
4 Dhingra N, Diepart M, Dziekan, et al. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva, Australia. 2010.
5 iData Research Inc. Global Market Report Suite for Vascular Access Devices and Accessories. 2020.
6 Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015; 38(3):189-203.
7 Twibell KR, Hofstetter P, Siela D, Brown D, Jones HM. A comparative study of blood sampling from venipuncture and short peripheral catheters in pediatric inpatients. J Infus Nurs. 2019;42(5):239. doi:10.1097/NAN.0000000000000338.
8 Mulloy DF, Lee SM, Gregas M, Hoffman KE, Ashley SW. Effect of peripheral IV based blood collection on catheter dwell time, blood collection, and patient response. Appl Nurs Res. 2018;40:76-79. doi:10.1016/j.apnr.2017.12.006.
9 Mannocci A, De Carli G, Di Bari V, et al. How much do needlestick injuries cost? A systematic review of the economic evaluations of needlestick and sharps injuries among healthcare personnel. Infect Control Hosp Epidemiol. 2016;37(6):636-646. doi:10.1017/ice.2016.48.
10 Mengistu, DA, Tolera ST, Demmu YM. Worldwide prevalence of occupational exposure to needle stick injury among healthcare workers: a systematic review and meta-analysis. Canadian Journal of Infectious Disease and Medical Microbiology. 2021.

| Last Trade: | US$225.15 |
| Daily Change: | -2.02 -0.89 |
| Daily Volume: | 1,682,996 |
| Market Cap: | US$65.080B |
November 07, 2024 October 30, 2024 October 29, 2024 October 14, 2024 September 26, 2024 | |

Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MORE
Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB