Annovis Bio, Inc. (NYSE: ANVS) ("Annovis" or the "Company"), a late-stage clinical drug platform company addressing neurodegenerative diseases, today released the following letter to stockholders from its Chief Executive Officer Dr. Maria Maccecchini.
Dear Fellow Stockholder,
Over the years we have been talking about the potential of buntanetap to treat more than Alzheimer's disease. In cell models, animal models and human clinical studies, we have seen that buntanetap works in numerous acute and chronic neurodegenerative conditions. I would like to take this opportunity to explain how one drug can be so broadly applicable.
Buntenatap works by inhibiting specific neurotoxic proteins such as amyloid precursor protein (APP), Tau, alpha-synuclein (αSYN), TAR DNA binding protein 43 (TDP-43), huntingtin (HTT) and prion protein. These proteins have normal functions but in their neurotoxic aggregating form, they impair axonal transport, slow synaptic transmission, cause inflammation, and ultimately, kill nerve cells, resulting in the loss of affected function in various neurodegenerative conditions.
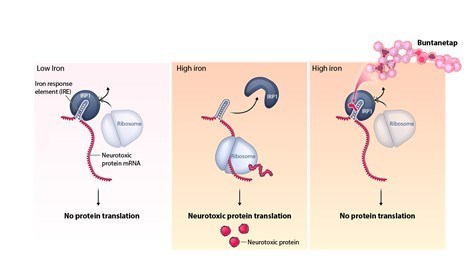
The overexpression and aggregation of these proteins is caused by elevated levels of iron in the nerve cell. The mRNAs of neurotoxic aggregating proteins contain an iron response element (IRE) which binds to an iron regulatory protein called IRP1. At normal iron levels, translation occurs at appropriate physiological levels. When iron flows into the cell, the mRNAs are released and translated at higher rates by the ribosome. When massive iron flows in, the mRNAs remain unbound for as long as the iron is high and the proteins for these neurotoxic aggregating proteins are overexpressed. In this high iron situation, buntanetap binds to the atypical IRE-IRP1 complex and prevents the mRNA from being released and, therefore, from being translated and overexpressed.
Buntanetap is able to inhibit the translation of multiple neurotoxic proteins through this mechanism of action, and as a result has the potential to treat numerous acute and chronic neurodegenerative conditions that share this pathway. In mouse or rat models of Alzheimer's, Parkinson's, stroke, frontotemporal dementia, traumatic brain injury, glaucoma, and Down Syndrome, buntanetap has been shown to normalize the levels of these neurotoxic proteins and to restore function.
Most importantly, this has been demonstrated in human clinical trials. In our recent Phase 2 clinical trial in Alzheimer's disease and Parkinson's disease, treatment with buntanetap resulted in reduction of aggregating proteins and statistically significant improvement in motor function in Parkinson's disease patients and cognition in Alzheimer's disease patients.
FUNCTION | TEST | SUBJECT |
ANIMALS | ||
Memory, learning | Mazes | AD mice, DS mice, stroke mice, TBI rats |
Movement | Colonic motility, grip strength | PD mice, tau mice |
Vision | Sight | Glaucoma rats |
Infections | Cell death | P. Gingivalis mice, Covid mice |
HUMANS | ||
Cognition, memory, learning | ADAScog11 | Early AD patients |
Attention, thinking speed | WAIS coding | Early AD patients |
Movement, coordination | MDS-UPDRS | Early PD patients |
Movement speed | WAIS coding | Early PD patients |
Table 1: Summary of all animal and human study data. | ||
We look forward to unlocking the full potential of buntanetap and addressing unmet needs across a range of acute and chronic neurological conditions.
Maria L. Maccecchini, Ph.D.,
Founder, President, CEO and Executive Board Member
About Buntanetap
Buntanetap (previously known as ANVS401 or Posiphen) is an oral translational inhibitor of neurotoxic aggregating proteins (TINAPs), which mode of action leads to lower levels of neurotoxic proteins and consequently less toxicity in the brain. In a Phase 2a clinical trial in AD and PD patients, treatment with buntanetap resulted in statistically significant improvement in motor function in PD patients and cognition in AD patients. Additionally, the drug was well-tolerated and safe, and its pharmacokinetics were found to be in line with levels measured earlier in humans, meeting both the primary and secondary endpoints.
About Annovis Bio, Inc.
Headquartered in Berwyn, Pennsylvania, Annovis Bio, Inc. is a late-stage clinical drug platform company developing transformative therapies that treat neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD) and other chronic and acute neurodegenerative diseases. The Company believes that it is the only company developing a drug that inhibits more than one neurotoxic protein, improves the information highway of the nerve cell, known as axonal transport, reduces inflammation and protects nerve cells from dying in chronic and acute neurodegeneration. Annovis conducted two Phase 2 studies: one in AD patients and one in both AD and PD patients. In the AD/PD study, buntanetap showed improvements in cognition and memory in AD as well as body and brain function in PD patients.
For more information on Annovis Bio, please visit the Company's website www.annovisbio.com and follow us on LinkedIn and Twitter.
Forward-Looking Statements
Statements in this press release contain "forward-looking statements" that are subject to substantial risks and uncertainties. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "expect," "believe," "will," "may," "should," "estimate," "project," "outlook," "forecast" or other similar words, and include, without limitation, statements regarding the timing, effectiveness, and anticipated results of buntanetap clinical trials. Forward-looking statements are based on Annovis Bio, Inc.'s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled "Risk Factors" in the Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission. Forward-looking statements contained in this announcement are made as of this date, and Annovis Bio, Inc. undertakes no duty to update such information except as required under applicable law.
Media and Investor Contact:
Nic Johnson
Russo Partners, LLC
(303) 482-6405
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$4.93 |
| Daily Change: | -0.18 -3.52 |
| Daily Volume: | 353,488 |
| Market Cap: | US$68.030M |
May 13, 2024 May 09, 2024 April 29, 2024 April 02, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB