BERWYN, Pa. / Nov 02, 2023 / Business Wire / Recent developments in biomarker research have enabled the performance of measurements of important biomarkers in plasma rather than in CSF (cerebrospinal fluid), making it possible to follow the changes in biomarkers during the course of a neurodegenerative disease while reducing patients’ burden.
TDP-43 (TAR DNA-binding protein 43) was discovered by Lee & Trojanowski at the University of Pennsylvania in 2007, and was suggested to be associated with frontotemporal dementia (FTD, 1),. Shaw’s lab in King’s College London associated TDP43 with amyotrophic lateral sclerosis published in Science 2008 (ALS, 2). More recently a number of papers in high profile journals have associated the protein with other neurodegenerative diseases, such as Alzheimer’s disease (AD, 3,4,5,8,9) and Parkinson’s disease (PD, 6,7). Of special interest, Dr. Ron Peterson from the Mayo Clinic pointed out in his 2018 Neurology review that protein abnormalities beyond amyloid and tau, including TDP-43 and α-synuclein, may contribute to neurodegeneration and cognitive impairment (5).
TDP-43 turns out to be a neurotoxic aggregating protein that has a normal function at normal levels, but when overexpressed it aggregates and becomes toxic, just like Aβ and α-synuclein. It impairs axonal transport, induces inflammation, and kills nerve cells.
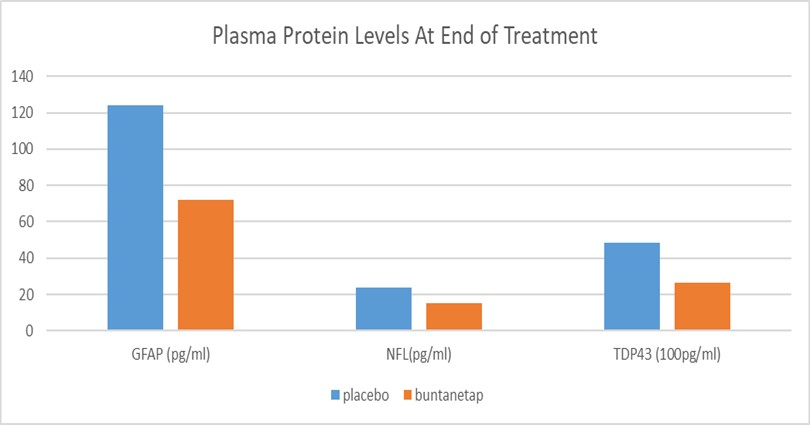
Two years ago, Annovis found that buntanetap inhibited expression of TDP43 in vitro through an unbiased proteomics search of proteins whose translation is regulated by buntanetap as published (10) and in table below. Now we were able to see a decrease of TDP43 in plasma of PD patients from our phase 2a study. This was made possible through the new Quanterix Simoa TDP43 assay, which detects both full-length and pathological, truncated forms of the protein. The data revealed that 80mg buntanetap treatment (n=10 patients) indeed reduced the accumulation of TDP43 in patients’ plasma by 71.7% compared to placebo (n=5 patients), with a statistically significant difference between placebo and treated p=0.05. Buntanetap also showed a trend in reducing inflammatory factor GFAP (Glial fibrillary acidic protein) and the axonal damage biomarker NfL (Neurofilament-light chain).
Table shows changes in protein levels of neurotoxic proteins whose mRNAs contain an atypical IRE (iron-responsive-element) and control proteins whose mRNAs contain a canonical IRE (FTL, and FHL1) in their 5′-UTR following buntanetap treatment of SH-SY5Y cells. The column with the ratios shows by how much the protein level goes down after the cells have been treated with buntanetap. This data is from our unbiased proteomics study.
Symbol | Gene Name | After/Before Treatment |
HTT | Huntingtin | 0.479 |
APP | Amyloid beta protein | 0.728 |
TARDBP | TAR DNA-binding protein 43 (TDP43) | 0.761 |
SNCA | Alpha-synuclein | 0.779 |
FTL | Ferritin light chain | 0.978 |
FTH1 | Ferritin heavy chain | 0.975 |
These exciting new data support buntanetap’s unique mechanism of action of reducing over-expression of multiple neurotoxic proteins in disease situations, therefore, reducing inflammation and neuronal damage, and preserving the neuronal function.
“We are pleasantly surprised to see a statistically significant drop in TDP43 levels in just 10 patients and to see a strong trend in GFAP and NfL. To our knowledge this is the first time that a drug reduces the levels of TDP43 in humans, specifically here in PD patients,” said Dr. Maccecchini. “These biomarker data not only corroborate the mechanism of actions of buntanetap, but also provide a new way to stratify patients and understand their disease pathology. We will measure the above biomarkers in the plasma of the patients that are presently in our phase 3 PD study to show the effect our drug has on the course of the disease over a six-month period.”
| Last Trade: | US$4.93 |
| Daily Change: | -0.18 -3.52 |
| Daily Volume: | 353,488 |
| Market Cap: | US$68.030M |
May 13, 2024 May 09, 2024 April 29, 2024 April 02, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB