ABBOTT PARK, Ill., April 14, 2023 /PRNewswire/ -- Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration (FDA) has cleared a reader for its FreeStyle Libre® 3 integrated continuous glucose monitoring (iCGM) system, which features the world's smallest, thinnest and most discreet5 glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible.3
"Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living," said Jared Watkin, senior vice president for Abbott's diabetes care business. "The FreeStyle 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems."
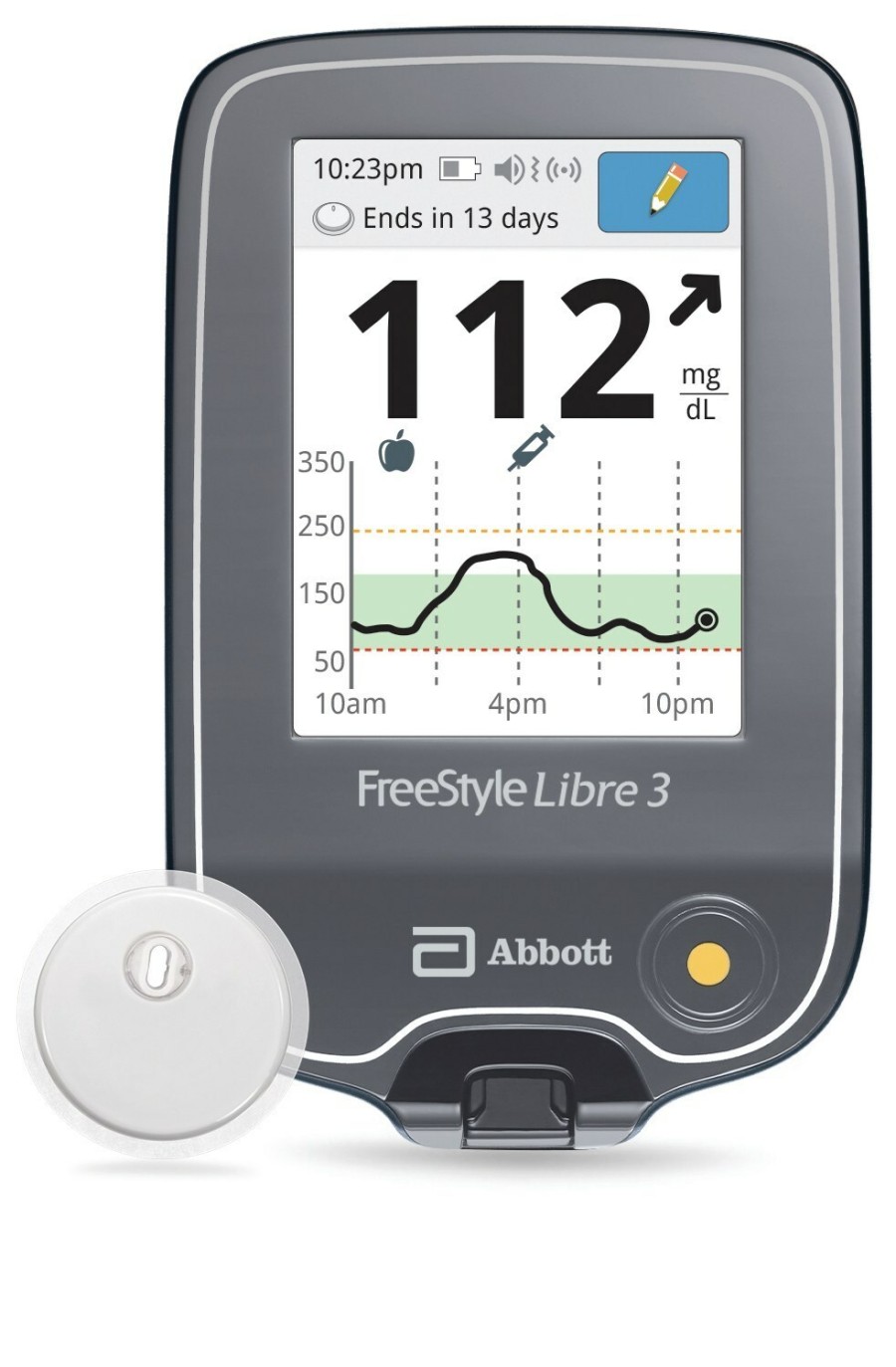
The FreeStyle Libre 3 reader is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings6 on a large, bright and easy-to-see screen.
People who use the FreeStyle Libre 3 system will still have the option to use the current FreeStyle Libre 3 smartphone apps.7,8
The reader uses a rechargeable lithium-ion battery, which is commonly found in many other electronic devices like mobile phones. The user manual for the FreeStyle Libre 3 reader provides details on how to safely store, charge and use the device, including always using the Abbott-provided USB cable and power adapter.
The FreeStyle Libre portfolio is the number one sensor-based glucose monitoring system in the world9, having changed the lives of 4.5 million people across more than 60 countries10 by providing breakthrough technology that is accessible and affordable.2
Important Safety Information
Failure to use the FreeStyle Libre 3 system as instructed in labeling may result in missing a severe low or high glucose event and/or making a treatment decision, resulting in injury. If glucose alarms and readings do not match symptoms or expectations, use a fingerstick value from a blood glucose meter for treatment decisions. Seek medical attention when appropriate or contact Abbott at 855-632-8658 for safety info.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 115,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com, on LinkedIn at www.linkedin.com/company/abbott-, on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews.

| Last Trade: | US$115.93 |
| Daily Change: | -1.20 -1.02 |
| Daily Volume: | 5,021,882 |
| Market Cap: | US$200.560B |
October 24, 2024 October 16, 2024 October 10, 2024 September 05, 2024 September 04, 2024 | |

Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MORE
Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB