BOSTON / Nov 07, 2023 / Business Wire / Verastem Oncology, (Nasdaq: VSTM), a biopharmaceutical company committed to advancing new medicines for patients with cancer, today announced results of the first-ever LGSOC Patient Impact Survey that reveal the particular challenges with diagnosis, disease management and mental, physical, and emotional well-being experienced by people living with low-grade serous ovarian cancer (LGSOC)*.1 This multi-national, online survey was conducted by The Harris Poll on behalf of the LGSOC Patient Impact Advisory Committee – a global collaboration among patient advocacy groups, including STAAR Ovarian Cancer Foundation (STAAR), Cure Our Ovarian Cancer and the World Ovarian Cancer Coalition (Coalition) as well as leaders in the medical community – with the aim to better understand and address the needs of those living with this rare and difficult to treat disease.
LGSOC is a rare, highly recurrent ovarian cancer associated with slow but persistent tumor growth and a high mortality rate and is known to be relatively resistant to chemotherapy.2,3,4 In 2014, LGSOC was classified as a separate disease compared to the more common high-grade serous ovarian cancer (HGSOC) by the World Health Organization, yet awareness and knowledge of the disease remains low.5 Eighty-five percent of people with LGSOC have their cancer come back after remission.4 Few treatment options are available for LGSOC and no treatments specifically for LGSOC have been approved to date.6
“Not only is LGSOC a rare disease, its classification as a distinct type of ovarian cancer was established within the last 10 years. With no medicines specifically approved by the U.S. Food and Drug Administration for LGSOC, along with limited research and published data about the patient experience and its impact, managing this disease is challenging for healthcare professionals and patients. This survey is an important collective effort to gather valuable insights and opinions to help better serve those living with LGSOC,” said Dr. David Gershenson, expert researcher in rare ovarian cancers and LGSOC Patient Impact Advisory Committee member. “As a physician dedicated to finding better ways to manage LGSOC, I witness how the long journey to diagnosis and the uncertainty of this disease takes a toll on my patients and their loved ones. These survey results underscore the urgency of educating on this unique form of ovarian cancer and provide important information to guide that effort.”
Delayed Diagnoses and Low Awareness
Results from the survey revealed the challenges people with LGSOC face in obtaining a timely and accurate diagnosis. Among the 81% of participants surveyed who experienced disease symptoms, it took an average of nearly three years to get an accurate diagnosis and most reported their path to diagnosis as difficult (73%) or frustrating (68%).1 Nearly seven-in-ten (68%) people surveyed who experienced symptoms, experienced a misdiagnosis and nearly two-thirds (66%) reported having their symptoms dismissed by a healthcare provider (HCP).1 Younger respondents (ages 18-49)** were more likely than their older peers (age 50+) to report that their symptoms were misdiagnosed (77% vs. 56%) and to feel that their symptoms were dismissed by their HCP (52% strongly agree vs. 32%).1 LGSOC often affects younger women compared to the more common high-grade serous ovarian cancer (HGSOC), with a median age at diagnosis between 45-55.3
Nearly all those surveyed (99%) had no awareness of LGSOC prior to diagnosis, and 60% did not feel that their HCP was “very knowledgeable” about this rare cancer.1 When it comes to educating about LGSOC, 87% want others to know that symptoms can be easily misdiagnosed as another disease, and 82% feel they should know that it’s important to listen to your body and seek medical attention as soon as possible.1
Challenges with Disease Management, Uncertainty and Anxiety
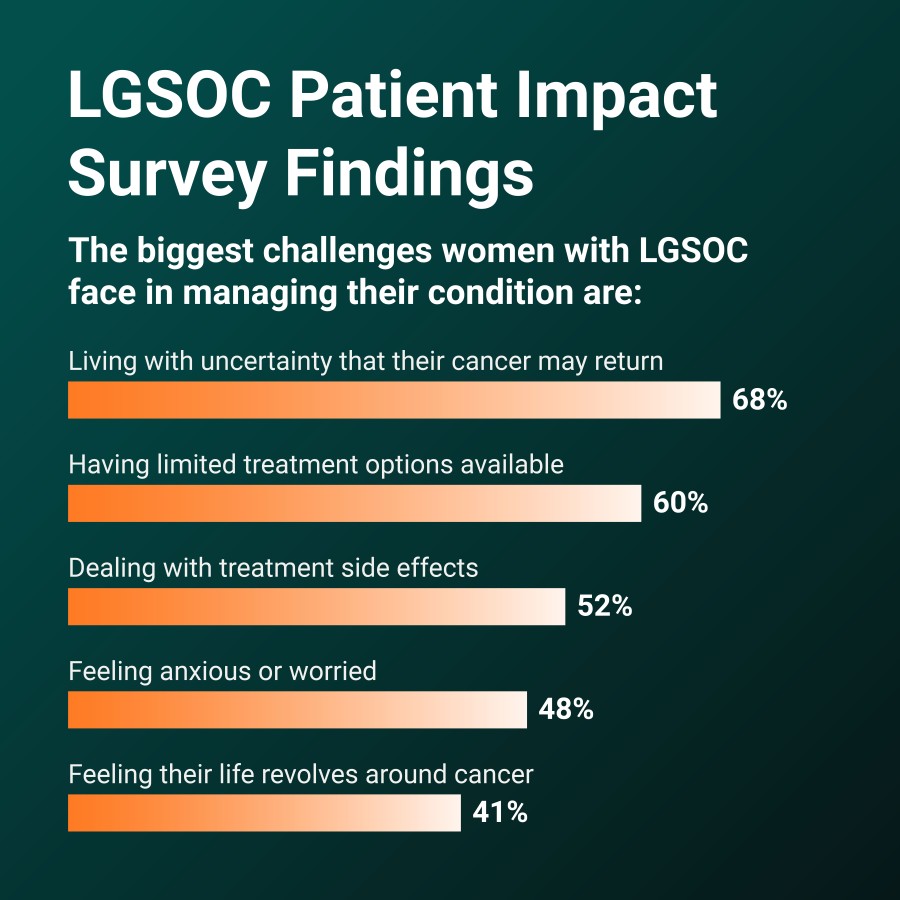
In the survey, 60% of respondents reported that one of the biggest challenges in managing their disease is that their treatment options are limited, with most (90%) feeling like they are getting the “treatment leftovers” instead of regimens studied in LGSOC.1 More than half (52%) reported experiencing side effects from treatment as a challenge and 25% reported having to pause or stop treatment because they couldn’t tolerate the side effects.1
The data also revealed the impact on respondents of navigating a recurrent disease with limited treatment options. Nearly seven-in-ten (68%) respondents reported that their biggest challenge is living with uncertainty that their cancer will return, more than two-in-five (41%) feel like their lives revolve around their cancer, and nearly half (48%) reported that their biggest challenge is experiencing anxiety or worry.1
“Since my LGSOC diagnosis, it has been so important to have my community around me. Although LGSOC is classified as a rare cancer, we have seen this community come together to provide support for each other, raise awareness of symptoms, and improve outcomes,” said Nicole Andrews, Board of Directors Chair, STAAR Ovarian Cancer Foundation and a member of the LGSOC Patient Impact Advisory Committee. “What this survey reveals is critical, because there are too many women like me who have had to navigate this experience on their own, with very few resources and low awareness of the disease. Having this measurable, actionable data that sheds new light on the challenges we face because of LGSOC is an important step toward education and awareness that can hopefully improve care.”
About the LGSOC Patient Impact Survey
The LGSOC Patient Impact Advisory Committee, supported by Verastem Oncology, worked with The Harris Poll to conduct a survey on behalf of leaders in the medical and advocacy communities. They surveyed 186 women ages 18+ who have been diagnosed with LGSOC across 10+ countries. The survey was conducted July 31st - August 29th, 2023. Raw data were not weighted and are therefore only representative of the individuals who completed the survey. Additional data from the survey were submitted to an upcoming medical congress.
The sampling precision of Harris online polls is measured by using a Bayesian credible interval. For this study, the sample data is accurate to within ± 7.1 percentage points using a 95% confidence level. This credible interval will be wider among subsets of the surveyed population of interest. All sample surveys and polls, whether or not they use probability sampling, are subject to other multiple sources of error which are most often not possible to quantify or estimate, including, but not limited to coverage error, error associated with nonresponse, error associated with question wording and response options, and post-survey weighting and adjustments.
To view survey details, including methodology, audience demographics, survey data and more, click here. Visit https://letstalkaboutlgsoc.com/resources/patient-impact-survey/ for more information.
*For the purposes of this survey, the term LGSOC refers to people diagnosed with low-grade serous carcinoma of the ovary or peritoneum (the thin layer of tissue lining the abdomen).
**Caution: small sample size (n<100). Results should be interpreted as directional.
About Low-Grade Serous Ovarian Cancer (LGSOC)
Low-grade serous ovarian cancer (LGSOC) is a highly recurrent, chemotherapy-resistant cancer, associated with slow tumor growth and high mortality rate.3 Approximately 6,000 women in the U.S. and 80,000 worldwide are living with this disease.7 Mutations in the KRAS gene are present in 30% of cases of LGSOC.8,9 LGSOC is most often diagnosed in women between the ages of 45-55 years and has a median survival of approximately ten years.7 The majority of patients experience severe pain and complications as the disease progresses. Chemotherapy is one of the preferred treatments for this disease, and there are limited treatment options currently available.7
About Verastem Oncology
Verastem Oncology (Nasdaq: VSTM) is a development-stage biopharmaceutical company committed to the development and commercialization of new medicines to improve the lives of patients diagnosed with cancer. Our pipeline is focused on novel small molecule drugs that inhibit critical signaling pathways in cancer that promote cancer cell survival and tumor growth, including RAF/MEK inhibition and focal adhesion kinase (FAK) inhibition. For more information, please visit www.verastem.com.
1 LGSOC Patient Impact Survey Research Findings. Harris Poll. 2023.
2 WHO Classification of Tumours, 5 Edition, Volume 4: Female Genital Tumours. https://publications.iarc.fr/592
3 Grisham, R. Low Grade Serous Carcinoma of the Ovary. Oncology. 2016. 30(7):650-652. https://www.cancernetwork.com/view/low-grade-serous-carcinoma-ovary. Accessed March 2023.
4 Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing Treatment in Recurrent Epithelial Ovarian Cancer. Expert Rev Anticancer Ther. 2017; 17:1147-1158. doi: 10.1080/14737140.2017.1398088.
5 Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumors of Female Reproductive Organs, 4th ed; 2014.
6 Ovarian Cancer Guidelines. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed October 2023.
7 Slomovitz B, Gourley C, Carey S. M, Malpica A, Shih I, Huntsman D, et al. Low-grade serous ovarian cancer: State of the science. Gynecol Oncol. 2020;156(3):715-725. https://doi.org/10.1016/j.ygyno.2019.12.033.
8 Timar J, Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer and Metastasis Reviews. 2020;39:1029–1038. https://doi.org/10.1007/s10555-020-09915-5.
9 Baines T. A, Xu D, Der C. J. Inhibition of RAS for cancer treatment: the search continues. Future Medicinal Chemistry. 2011;3(14):1787–1808. https://doi.org/10.4155/fmc.11.121.
| Last Trade: | US$4.19 |
| Daily Change: | -0.25 -5.63 |
| Daily Volume: | 1,731,555 |
| Market Cap: | US$186.500M |
December 18, 2024 November 06, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB