BRISBANE, Calif., Jan. 30, 2023 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. (Nasdaq: VERA), a late-stage biotechnology company focused on developing and commercializing transformative treatments for patients with serious immunological disease, today announced results from a prespecified per-protocol (PP) analysis of the Phase 2b ORIGIN clinical trial of atacicept in patients with IgA nephropathy (IgAN) following announcement of topline results on January 3, 2023. The prior topline results reflected the intent-to-treat (ITT) analysis of all randomized patients (n=116), which is a conservative assessment of efficacy. In the prespecified PP analysis, the population was defined as patients who had completed treatment according to protocol (n=102). 14 patients across treatment arms who had protocol violations were identified by a blinded third-party CRO and excluded, for prespecified reasons as outlined in Figure 1.
Figure 1. Patients Identified by Blinded Third-Party CRO and Excluded from Prespecified PP Analysis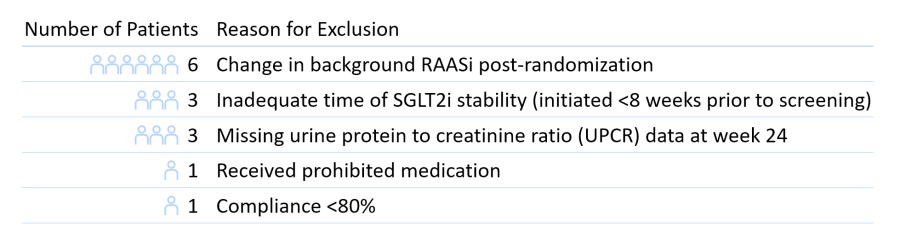
Atacicept is the Company’s potential best-in-class, disease-modifying dual inhibitor of the cytokines B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL). ORIGIN is a multinational, randomized, double-blind, placebo-controlled clinical trial evaluating the efficacy and safety of atacicept in patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite available ACE or ARB therapy.
“This new analysis of proteinuria reduction in the ORIGIN Phase 2b clinical trial shows atacicept's ability to substantially reduce proteinuria, independent of changes in background regimens in the context of a multinational, randomized, placebo-controlled trial,” said Jonathan Barratt, Ph.D., FRCP, The Mayer Professor of Renal Medicine, University of Leicester, U.K.
“We believe the PP analysis represents a more accurate assessment of treatment efficacy when compared to the ITT analysis, as it minimizes potential confounders for proteinuria measure. Our confidence in the promising clinical potential of atacicept is further bolstered by this analysis and we are rapidly advancing atacicept’s development as a potentially transformative therapy for patients with IgAN,” said Marshall Fordyce, M.D., Chief Executive Officer of Vera Therapeutics. “This analysis, as well as certain subgroup analyses, will help to inform the design and management of the Phase 3 clinical trial. Our team is well positioned to begin the pivotal trial in the first half of the year, subject to and following discussions with the FDA. With current timelines, we expect to announce Week 36 topline data from the Phase 3 clinical trial in the first half of 2025. Pending the data from the Phase 3 clinical trial, we expect to submit a BLA for atacicept to the FDA in the second half of 2025.”
Prespecified Per-Protocol (PP) Analysis from the Phase 2b ORIGIN Clinical Trial
In the topline results published on January 3, 2023, all treated patients (n=116) were included in the results as the intent-to-treat (ITT) population. In the prespecified PP analysis, the population was defined as patients who had completed treatment according to protocol (n=102), where 14 patients who had protocol violations identified by a blinded third-party CRO were excluded. These protocol violations are outlined above in Figure 1.
In the PP analysis, at Week 24, the atacicept 150 mg dose group achieved a 41% mean reduction in proteinuria versus baseline and a 34% delta versus placebo (p=0.025). With interim data at Week 36, the atacicept 150 mg dose group achieved a 47% mean reduction in proteinuria from baseline and a 48% delta versus placebo, as shown in Figure 2. Data for the atacicept 150 mg dose group versus placebo from both the PP and ITT analyses can be referenced in Figure 3.
Figure 2. Prespecified PP Analysis: UPCR % Change In Atacicept 75 and 150 mg Through Week 36
Figure 3. Summary of Positive Phase 2b Results (PP Analysis, ITT Analysis)
Safety results indicated that atacicept was generally well-tolerated and were consistent with the previously observed safety profile of atacicept, including a 1% discontinuation rate due to adverse events (AEs) and comparable rates of infection compared to placebo. Serious treatment-emergent AEs were observed in 2% of patients in all atacicept arms and in 9% of patients in the placebo arm. These results build upon the prior integrated analysis of atacicept in randomized, double-blind, placebo-controlled clinical trials in over 1,500 patients to date – in which atacicept was well-tolerated.
Next Steps
Vera is continuing to rapidly advance atacicept into pivotal Phase 3 development, which is anticipated in the first half of 2023, subject to and following discussions with the FDA. The full data sets from the ORIGIN clinical trial will be presented at upcoming medical congresses. Vera plans to prioritize and focus current resources on the advancement of atacicept in IgAN into a pivotal Phase 3 trial, extending cash runway to the fourth quarter of 2024. This updated cash runway guidance assumes a delay in enrollment in the pivotal Phase 3 trial for lupus nephritis, and a delay of commitment of resources to the MAU868 program until regulatory agreement is reached regarding the pivotal Phase 3 program for the treatment of BK viremia in kidney transplant recipients
Conference Call / Webcast Details
Vera will host a conference call and webcast with slide presentation at 8:00 a.m. ET on January 30, 2023. The live webcast will be available here, and on the Events & Presentations page of the Vera website, with the recording and presentation available immediately following the event.
About the ORIGIN clinical trial
The ORIGIN clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled Phase 2b trial evaluating the safety and efficacy of atacicept in 116 patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of RAASi for at least 12 weeks that is the maximum labeled or tolerated dose.
The objectives of the study are to determine the effect of atacicept on proteinuria and preservation of renal function compared to placebo to determine the appropriate dose(s) for further clinical development.
The primary endpoint is the change in proteinuria as evaluated by UPCR at week 24 and the key secondary endpoint is the change in proteinuria as evaluated by UPCR at week 36.
Additional exploratory endpoints include change in proteinuria as evaluated by UPCR at weeks 12, 48, and 96; change in estimated glomerular filtration rate (eGFR); change in serum immunoglobulin levels, and serum Gd-IgA1 levels; safety and tolerability; and serum pharmacokinetics (PK).
The ORIGIN clinical trial evaluated three dose strengths of atacicept versus placebo, administered weekly by prefilled syringe, and their impact on the reduction of proteinuria as evaluated by urine protein to creatinine ratio (UPCR). Patients were randomized 2:2:1:2 to atacicept 150 mg, atacicept 75 mg, atacicept 25 mg, or matching placebo. Upon completion of the 36-week blinded treatment period, all patients are being offered open-label atacicept 150 mg for an additional 60 weeks. For more information about the ORIGIN clinical trial, please visit www.clinicaltrials.gov.
About IgA nephropathy (IgAN), or Berger’s disease
IgAN, also known as Berger’s disease, is a serious and progressive autoimmune disease of the kidney, for which there remains a high unmet medical need. IgAN is driven by the production of immunogenic galactose-deficient IgA1 (Gd-IgA1), which triggers autoantibodies that lead to the formation of pathogenic immune complexes, which become trapped in the kidney’s glomeruli, causing inflammation and progressive damage. In up to 50 percent of patients, IgAN can lead to end-stage renal disease (ESRD) or kidney failure, which has considerable morbidity and impact on patients’ lives.
About Atacicept
Atacicept is an investigational recombinant fusion protein that contains the soluble transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) receptor that binds to the cytokines B lymphocyte stimulator (BlyS) and a proliferation-inducing ligand (APRIL). These cytokines are members of the tumor necrosis factor family that promote B-cell survival and autoantibody production associated with certain autoimmune diseases, including IgA nephropathy and lupus nephritis. Atacicept showed a dose-dependent effect on key biomarkers and clinical markers in a Phase 2a clinical study. Vera believes atacicept is positioned for best-in-class potential, targeting B cells and plasma cells to reduce autoantibodies and having been administered to more than 1,500 patients in clinical studies across different indications.
About Vera
Vera Therapeutics is a late-stage biotechnology company focused on developing treatments for serious immunological diseases. Vera’s mission is to advance treatments that target the source of immunologic diseases in order to change the standard of care for patients. Vera’s lead product candidate is atacicept, a fusion protein self-administered as a subcutaneous injection once weekly that blocks both B lymphocyte stimulator (BLyS) and a proliferation inducing ligand (APRIL), which stimulate B cells and plasma cells to produce autoantibodies contributing to certain autoimmune diseases, including IgA nephropathy (IgAN), also known as Berger’s disease, and lupus nephritis. In addition, Vera is evaluating additional diseases where the reduction of autoantibodies by atacicept may prove medically useful. Vera is also developing MAU868, a monoclonal antibody designed to neutralize infection with BK Virus, a polyomavirus that can have devastating consequences in certain settings such as kidney transplant. Vera retains all global developmental and commercial rights to atacicept and MAU868. For more information, please visit www.veratx.com.
Forward-looking Statement
Statements contained in this press release regarding matters, events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, atacicept’s potential to be a transformational treatment for patients with IgAN and a best-in-class therapy, Vera’s plans to advance atacicept into pivotal Phase 3 development in the first half of 2023 and the design and management of such trial, expectations regarding reporting Phase 3 topline data at Week 36, regulatory matters, including the timing and likelihood of success in obtaining drug approvals, including a potential BLA submission in the second half of 2025, Vera’s plans to prioritize and focus current resources on the advancement of atacicept in IgAN into a pivotal Phase 3 trial, and extending cash runway to the fourth quarter of 2024, including the assumptions underlying such guidance. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “advance,” “anticipate,” “could,” “expect,” “look,” “will,” “potential,” “plan,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Vera’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks related to the regulatory approval process, results of earlier clinical trials may not be obtained in later clinical trials, risks and uncertainties associated with Vera’s business in general, the impact of macroeconomic and geopolitical events, including the COVID-19 pandemic, and the other risks described in Vera’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Vera undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Contacts
Investor Contact:
Joyce Allaire
LifeSci Advisors
212-915-2569
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Contact:
Uncapped Communications
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$43.92 |
| Daily Change: | 0.01 0.02 |
| Daily Volume: | 5,315 |
| Market Cap: | US$2.780B |
November 11, 2024 November 07, 2024 October 02, 2024 | |

Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MORE
Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB