CHATHAM, N.J., Dec. 20, 2023 (GLOBE NEWSWIRE) -- Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a biopharmaceutical company with marketed products and a pipeline of development candidates, today announced that the Phase 3 RESILIENT study evaluating TNX-102 SL (cyclobenzaprine HCl sublingual tablets) met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials, significantly reducing daily pain compared to placebo (p=0.00005) in participants with fibromyalgia (Table 1). Statistically significant and clinically meaningful results were also seen in all key secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Additionally, as it relates to improving daily pain, treatment with TNX-102 SL showed a robust and clinically meaningful analgesic effect size of 0.38, with rapid onset of action, separating from placebo for each week of the study. TNX-102 SL was well tolerated with an adverse event profile comparable to prior studies, and no new safety signals were observed. Tonix plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2024 for TNX-102 SL for the management of fibromyalgia. An estimated 6 million to 12 million U.S. adults are living with fibromyalgia, the majority of whom are women.
TNX-102 SL is a tablet formulation containing 2.8 mg cyclobenzaprine HCl and is a novel, centrally-acting, non-opioid analgesic, designed to be taken once daily at bedtime for the management of fibromyalgia. RESILIENT was a 14-week randomized, double-blind, placebo-controlled trial of TNX-102 SL 5.6 mg, in which 457 participants with fibromyalgia were randomized in a 1:1 ratio to TNX-102 SL or placebo across 33 sites in the U.S. All participants received one 2.8 mg tablet of TNX-102 SL (2.8 mg) or placebo for the first 2 weeks, which was increased to two 2.8 mg tablets of TNX-102 SL (5.6 mg) or placebo for the remaining 12 weeks.
In December 2020, Tonix reported positive results from the first Phase 3 RELIEF study of TNX-102 SL 5.6 mg for the management of fibromyalgia. The RELIEF study met its pre-specified primary endpoint, significantly reducing daily pain compared to placebo (p=0.010) in participants with fibromyalgia, and showing activity in key secondary endpoints.
“We believe that the positive results of RESILIENT and RELIEF show that fibromyalgia can be successfully treated by TNX-102 SL 5.6 mg and may provide the opportunity for Tonix to have the first FDA-approved drug for fibromyalgia in more than a decade,” said Seth Lederman, M.D., President and Chief Executive Officer of Tonix Pharmaceuticals. “We are now an important step closer to bringing a new, first-line treatment to fibromyalgia patients that offers broad symptom relief and favorable tolerability for chronic use and adherence. We believe that we are well positioned to submit an NDA to the FDA under the 505(b)(2) regulatory approval pathway in the second half of 2024, and are on track to supply the U.S. market upon FDA approval.”
Table 1. Results of Primary and Secondary Endpoints for the Phase 3 RESILIENT Study of TNX-102 SL
| Outcome Measure at Week 14 | Intent-to-Treat Analysis1 | P-value | ||||
| Primary Endpoint | ||||||
| Daily Pain Diary, NRS | Mean Change from Baseline2 | 0.00005* | ||||
| Key Secondary Endpoints | ||||||
| Non-specific | ||||||
| Patient Global Impression of Change | Proportion "Much" or "Very Much Improved"3 | <0.001* | ||||
| Fibromyalgia Syndrome-Related | ||||||
| FIQ-R Symptom Domain | Mean Change from Baseline | <0.001* | ||||
| FIQ-R Function Domain | Mean Change from Baseline | 0.001* | ||||
| PROMIS Sleep Disturbance | Mean Change from Baseline | <0.001* | ||||
| PROMIS Fatigue | Mean Change from Baseline | <0.001* | ||||
| Daily Sleep Quality Diary, NRS | Mean Change from Baseline | <0.001* | ||||
| Abbreviations: FIQ-R = Fibromyalgia Impact Questionnaire - Revised; NRS = Numeric Rating Scale; PROMIS = Patient-Reported Outcomes Measurement Information System | ||||||
| *Statistically significant; to control for overall type 1 error, a pre-specified, serial gatekeeping procedure was utilized. | ||||||
| 1Analysis by mixed model repeated measures with multiple imputation unless otherwise indicated. | ||||||
| 2Primary endpoint analysis for FDA approvals of Cymbalta® and Lyrica® in fibromyalgia. | ||||||
| 3Pearson’s chi-squared test responder analysis, with missing data considered non-responders | ||||||
“These data are terrific news for patients with fibromyalgia,” said Daniel J. Clauw, M.D., Professor of Anesthesiology, Medicine and Psychiatry at the University of Michigan. “Despite approved medications, there remains a need for new treatment options to better address the quality of life impacts many fibromyalgia patients experience on a chronic basis. TNX-102 SL is a non-opioid, centrally-acting analgesic, the active ingredient of which has a known, favorable safety profile from decades of use. The fact that cyclobenzaprine was also beneficial in many other key symptom domains, including sleep quality, sleep disturbance and fatigue, will be appreciated by fibromyalgia patients that struggle with not just pain but multiple other symptoms.”
“These positive data from RESILIENT and previously with RELIEF, with remarkable separation from placebo on pain, sleep, and fatigue, add support to TNX-102 SL’s proposed mechanism of improving sleep quality to improve the syndromal effects of fibromyalgia,” commented Gregory Sullivan, M.D., Chief Medical Officer of Tonix Pharmaceuticals. “The sublingual formulation of TNX-102 SL, which uses our proprietary Protectic® and Angstro® technologies, is integral to our treatment paradigm. These technologies enable transmucosal delivery of cyclobenzaprine with distinctive pharmacokinetic properties that include rapid absorption after dosing and bypass of first-pass hepatic metabolism. I would like to thank the RESILIENT study participants and their families and caregivers, as well as the investigators and their hard-working staff who all made this a highly successful trial.”
Summary of Topline Results of the RESILIENT Study
The RESILIENT study achieved statistical significance on the pre-specified primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX-102 SL 5.6 mg (LS mean [SE]: -1.8 [0.12] units) versus placebo (-1.2 [0.12] units), analyzed by mixed model repeated measures with multiple imputation (LS mean [SE] difference: -0.7 [0.16] units, p=0.00005, Table 1). In addition, all pre-specified sensitivity analyses of the primary endpoint were statistically significant (p<0.001). Figure 1 shows reduction in pain across all weeks of the 14-week study, with nominal p<0.01 for every week. Note the rapid onset of action with separation from placebo at Week 1 was sustained throughout all weeks of dosing.
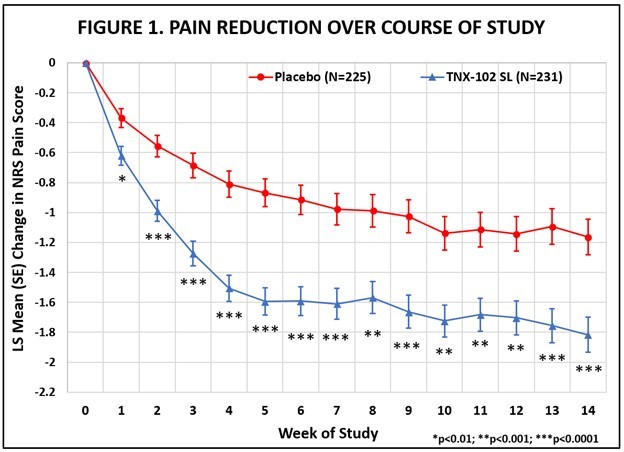
Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error
The statistically significant improvement in pain is further substantiated when diary pain was analyzed by another standard statistical approach, a 30 percent responder analysis, with 45.9% on active and 27.1% on placebo having a 30 percent or greater reduction in pain (Pearson Chi-Squared Test; difference in proportions [95% CI]: 18.8% [10.1%, 27.4%]; nominal p<0.001).
TNX-102 SL showed statistical significance (p≤0.001) on all six pre-specified key secondary efficacy outcome measures (Table 1).
Consistent with the proposed mechanism that TNX-102 SL acts in fibromyalgia through improving sleep quality, TNX-102 SL showed statistically significant improvement of sleep by two main measures. For the daily diary sleep quality ratings, improvement in sleep quality for TNX-102 SL (-1.8 [0.12] units) was significantly greater than that of placebo (-1.2 [0.12] units; LS mean [SE] difference from placebo: -0.6 [0.17] units; p<0.001). For the PROMIS Sleep Disturbance instrument, TNX-102 SL also demonstrated significantly greater improvement over placebo on T-scores (LS mean [SE] difference from placebo: -4.2 [0.79] units; p<0.001). Fatigue is another cardinal symptom of fibromyalgia and has a major impact on quality of life. TNX-102 SL showed significant improvement over placebo on the PROMIS Fatigue instrument T-scores (-3.0 [0.77] units; p<0.001).
The Fibromyalgia Impact Questionnaire – Revised (FIQ-R) is a 21-item self-rated instrument that assesses level of function, overall impact, and symptoms due to fibromyalgia, and the symptoms and function domains were key secondary endpoints in RESILENT. At Week 14 on the FIQ-R Symptoms domain, there was significantly greater improvement with TNX-102 SL than with placebo (LS mean [SE] difference from placebo: -7.7 [1.62], p<0.001). Similarly, TNX-102 SL resulted in greater improvement on FIQ-R Function (LS mean [SE] difference from placebo: -5.4 [1.66], p=0.001). Although not a key secondary efficacy endpoint, TNX-102 SL also separated from placebo on the FIQ-R Impact domain (nominal p=0.001). These results, along with the robust effects on improving sleep and fatigue, suggests broad symptomatic coverage of the syndrome of fibromyalgia.
Safety Results of the Phase 3 RESILIENT Study
In the RESILIENT study, TNX-102 SL was well tolerated and consistent with prior trials, with no new safety signals observed. Among participants randomized to the TNX-102 SL and placebo arms, 81.0% and 79.2%, respectively, completed the 14-week dosing period. As expected based on prior TNX-102 SL studies, administration site reactions were the most commonly reported adverse events and were higher in the TNX-102 SL treatment group (Table 2). Hypoaesthesia oral and paraesthesia oral, or tongue and mouth numbness or tingling, product taste abnormal (typically a bitter aftertaste upon dosing), and tongue discomfort were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. The only treatment-emergent adverse events that occurred at a rate of 3.0% or greater in either arm were these four oral adverse events, along with COVID-19, somnolence, and headache (Table 2). Adverse events resulted in premature study discontinuation in 6.1% of those who received TNX-102 SL compared with 3.5% of placebo recipients. There were a total of seven serious adverse events in five patients, five of which were experienced by three patients in the placebo arm, and two of which were in the TNX-102 SL arm. Of the two in the TNX-102 SL arm, one was renal cancer, deemed unrelated to study drug, and the other was acute pancreatitis with onset 14 days after dosing was completed and reported as possibly related to study drug.
Table 2. Treatment-Emergent Adverse Events at a Rate of 3% or Greater in Either Treatment Arm
| TNX-102 SL (N=231) | Placebo (N=226) | Total (N=457)* | ||||||||||
| Administration Site Reactions | N | % | N | % | N | % | ||||||
| Hypoaesthesia oral | 55 | 23.8% | 1 | 0.4% | 56 | 12.3% | ||||||
| Product taste abnormal | 27 | 11.7% | 2 | 0.9% | 29 | 6.3% | ||||||
| Paraesthesia oral | 16 | 6.9% | 2 | 0.9% | 18 | 3.9% | ||||||
| Tongue discomfort | 16 | 6.9% | 0 | 0.0% | 16 | 3.5% | ||||||
| Systemic | ||||||||||||
| Adverse Events | N | % | N | % | N | % | ||||||
| COVID-19 | 10 | 4.3% | 7 | 3.1% | 17 | 3.7% | ||||||
| Somnolence | 7 | 3.0% | 3 | 1.3% | 10 | 2.2% | ||||||
| Headache | 7 | 3.0% | 4 | 1.8% | 11 | 2.4% | ||||||
*Safety Population
The Changes in Sexual Functioning Questionnaire short form (CSFQ-14) served as a safety measure for assessing potential adverse effects on sexual functioning. In females, the total score on the CSFQ-14 at Week 14 improved (indicating better sexual functioning) in the TNX-102 SL group compared with placebo (nominal p=0.010 by analysis of covariance). This potentially indicates an important tolerability advantage over pharmacotherapeutics which potently inhibit reuptake of serotonin. The low percentage of males in the safety population (<5%) did not allow meaningful analysis of the CSFQ-14 data.
About the Phase 3 RESILIENT Study
The RESILIENT study is a double-blind, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of TNX-102 SL (cyclobenzaprine HCl sublingual tablets) in the management of fibromyalgia. The two-arm trial randomized 457 participants in the U.S. across 33 sites. The first two weeks of treatment consist of a run-in period in which participants start on TNX-102 SL 2.8 mg (1 tablet) or placebo. Thereafter, all participants increase their dose to TNX-102 SL 5.6 mg (2 x 2.8 mg tablets) or two placebo tablets for the remaining 12 weeks. The primary endpoint is the daily diary pain severity score change (TNX-102 SL 5.6 mg vs. placebo) from baseline to Week 14 (using the weekly averages of the daily numerical rating scale scores), analyzed by mixed model repeated measures with multiple imputation.
For more information, see ClinicalTrials.gov Identifier: NCT05273749.
About Fibromyalgia
Fibromyalgia is a chronic pain disorder that is understood to result from amplified sensory and pain signaling within the central nervous system. Fibromyalgia afflicts an estimated 6 million to 12 million adults in the U.S., the majority of whom are women. Symptoms of fibromyalgia include chronic widespread pain, nonrestorative sleep, fatigue, and morning stiffness. Other associated symptoms include cognitive dysfunction and mood disturbances, including anxiety and depression. Individuals suffering from fibromyalgia struggle with their daily activities, have impaired quality of life, and frequently are disabled. Physicians and patients report common dissatisfaction with currently marketed products.
About TNX-102 SL
TNX-102 SL is a patented sublingual tablet formulation of cyclobenzaprine hydrochloride which provides rapid transmucosal absorption and reduced production of a long half-life active metabolite, norcyclobenzaprine, due to bypass of first-pass hepatic metabolism. As a multifunctional agent with potent binding and antagonist activities at the 5-HT2A-serotonergic, α1-adrenergic, H1-histaminergic, and M1-muscarinic cholinergic receptors, TNX-102 SL is in development as a daily bedtime treatment for fibromyalgia, fibromyalgia-type Long COVID (formally known as post-acute sequelae of COVID-19 [PASC]), alcohol use disorder and agitation in Alzheimer’s disease. Dr. Harvey Moldofsky, Professor Emeritus of Psychiatry and Medicine at the University of Toronto, founding Director of the University of Toronto Center for Sleep and Chronobiology, first recognized the central role of non-restorative sleep in the pathogenesis of fibromyalgia1,2. Our program is based on the subsequent pioneering work of Dr. Iredell W. Iglehart III, who recognized that a sleep-focused cyclobenzaprine treatment protocol had the potential to target non-restorative sleep and lead to improvement of fibromyalgia at the syndromal level3. Teams led by Giorgio Reiner at APR Applied Pharma Research S.A., a wholly-owned subsidiary of Relief Therapeutics Holding AG, and Professor Marino Nebuloni and Patrizia Colombo at Redox Analytical Science Srl invented and developed these underlying technologies in collaboration with Tonix. The United States Patent and Trademark Office (USPTO) issued United States Patent No. 9636408 in May 2017, Patent No. 9956188 in May 2018, Patent No. 10117936 in November 2018, Patent No. 10,357,465 in July 2019, and Patent No. 10736859 in August 2020. The Protectic™ protective eutectic and Angstro-Technology™ formulation claimed in the patent are important elements of Tonix’s proprietary TNX-102 SL composition. These patents are expected to provide TNX-102 SL, upon NDA approval, with U.S. market exclusivity until 2034/2035. In addition, Tonix has pending but not issued U.S. patent applications directed to the transmucosal absorption of CBP-HCl, with U.S. market exclusivity expected until 2033, for treating major depressive disorder in fibromyalgia, with U.S. market exclusivity expected until 2032, and for treating pain in fibromyalgia with U.S. market exclusivity expected until 2041.
1Moldofsky H et al, Psychosom Med 1975;37:341-51.
2Moldofsky H and Scarisbrick P. Psychosom Med 1976;38:35-44.
3Iglehart IW. 2003; US Patent 6,541,523.
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix Medicines, our commercial subsidiary, markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg under a transition services agreement with Upsher-Smith Laboratories, LLC from whom the products were acquired on June 30, 2023. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead development CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), has completed two positive Phase 3 studies for the management of fibromyalgia. Tonix intends to meet with the FDA and submit an NDA for the approval of TNX-102 SL for the management of fibromyalgia in the second half of 2024. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition, and topline results were reported in the third quarter of 2023. TNX-1900 (intranasal potentiated oxytocin) is being studied in binge eating disorder, pediatric obesity, bone health in autism and social anxiety disorder by academic collaborators under investigator-initiated INDs. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the first quarter of 2024 Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 was initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases, including TNX-1800, in development as a vaccine to protect against COVID-19. During the fourth quarter of 2023, TNX-1800 was selected by the U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) Project NextGen for inclusion in Phase 1 clinical trials. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, which are classes of broad-spectrum small molecule oral antivirals.
*Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines. All other marks are property of their respective owners.
This press release and further information about Tonix can be found at www.tonixpharma.com.
About Redox - Analytical Science Srl
Redox is an independent CRO company headquartered in Monza- Italy with R&D activities and customer analytical support to pharmaceutical companies for more than 30 years. From more than 25 years the analytical activities have been certified by national and international agencies (European Medicines Agency, the Italian Medicines Agency (AIFA), FDA, and etc). One of the main activities is the development of new drug products in order to improve the pharmaceutical actions and at the same time improve the stability and reducing the cost of the new drug substance. Several unique and sophisticated analytical techniques and equipment are used in support to research and development strategies with the focus to reach the best and effective pharmaceutical formulation in a short time frame. More than 30 professional people are dedicated to our efforts and many projects are ongoing in collaboration with the pharmaceutical industry as well as with Italian and international Universities.
Further information about Redox can be found at www.labredox.com.
About APR Applied Pharma Research S.A., a wholly-owned subsidiary of Relief Therapeutics Holding AG
Relief Therapeutics is a commercial-stage biopharmaceutical company committed to advancing treatment paradigms and delivering improvements in efficacy, safety, and convenience to benefit the lives of patients living with select specialty and rare diseases. Relief Therapeutics' portfolio offers a balanced mix of marketed, revenue-generating products, our proprietary, globally patented Physiomimic™ and TEHCLO™ platform technologies and a targeted clinical development pipeline consisting of risk-mitigated assets focused in three core therapeutic areas: rare metabolic disorders, rare skin diseases and rare respiratory diseases. In addition, Relief Therapeutics is commercializing several legacy products via licensing and distribution partners. Relief Therapeutics' mission is to provide therapeutic relief to those suffering from rare diseases and is being advanced by an international team of well-established, experienced biopharma industry leaders with extensive research, development and rare disease expertise. Relief Therapeutics is headquartered in Geneva, with additional offices in Balerna, Switzerland, Offenbach am Main, Germany and Monza, Italy. Relief Therapeutics is listed on the SIX Swiss Exchange under the symbol RLF.
Further information about APR can be found at www.relieftherapeutics.com or by following Relief Therapeutics on LinkedIn and Twitter.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
This email address is being protected from spambots. You need JavaScript enabled to view it.
(862) 904-8182
Peter Vozzo
ICR Westwicke
This email address is being protected from spambots. You need JavaScript enabled to view it.
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
This email address is being protected from spambots. You need JavaScript enabled to view it.
443-213-0495

| Last Trade: | US$0.37 |
| Daily Change: | -0.02 -3.97 |
| Daily Volume: | 12,731,124 |
| Market Cap: | US$69.150M |
December 23, 2024 December 17, 2024 December 03, 2024 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB