- Deployed new digital chemistry technology, together with NVIDIA, to predict the ligand-protein interactions for approximately 36 billion compounds in the Enamine REAL Space, reported to be the world’s largest searchable chemical library
- Added, accelerated or tightened guidance for clinical study readouts or clinical starts; the first clinical readout is expected in Q3 2023
- Delivered value in our pipeline, partnerships, and platform through the acquisitions of Cyclica and Valence Discovery
- Announced a $50 million investment and partnership with NVIDIA to accelerate the construction, optimization and deployment of foundation models for biology and chemistry
- Continued advancing large-scale foundation models for drug discovery with our partners at NVIDIA
SALT LAKE CITY, Aug. 08, 2023 (GLOBE NEWSWIRE) -- Recursion (Nasdaq: RXRX), a leading clinical stage TechBio company decoding biology to industrialize drug discovery, today reported business updates and financial results for its second quarter ending June 30, 2023.
"As the world continues to undergo a revolution in artificial intelligence and computation, Recursion is leading the TechBio sector with one of the most advanced technology-enabled drug discovery platforms in the industry," said Chris Gibson, Ph.D., Co-Founder and CEO of Recursion. "Our recent acquisitions of Cyclica and Valence and our new partnership with NVIDIA bring industry-leading capabilities to our platform that have already delivered significant value across our pipeline and partnerships. With multiple clinical catalysts in the coming quarters, the extraordinary progress in our technology, and the commitment of our teams, Recursion is making its vision of industrialized drug discovery real day by day."
Summary of Business Highlights
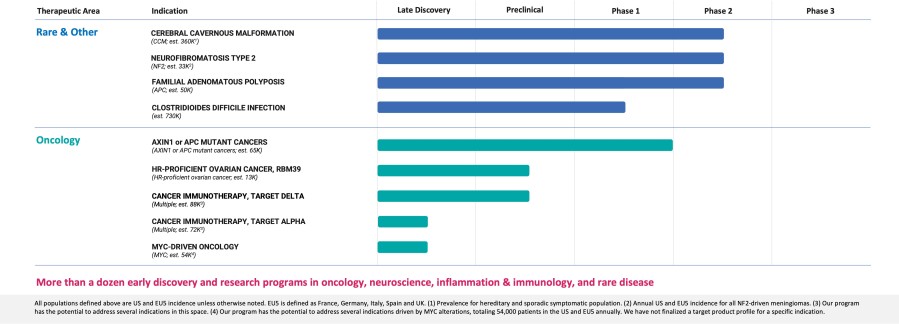
- Pipeline
- Cerebral Cavernous Malformation (CCM) (REC-994): Our Phase 2 SYCAMORE clinical trial is a double-blind, placebo-controlled safety, tolerability and exploratory efficacy study of this drug candidate in participants with CCM. This study was fully enrolled as of June 2023 with 62 participants and all participants who have thus far finished their first year of treatment have enrolled in the long-term extension study. We expect to share Phase 2 proof-of-concept data in H2 2024.
- Neurofibromatosis Type 2 (NF2) (REC-2282): Our Phase 2/3 POPLAR clinical trial is a two part study of REC-2282 in participants with progressive NF2-mutated meningiomas. Part A of the study is ongoing and is exploring two doses of REC-2282 in approximately 23 adults and 9 adolescents. We expect to share Phase 2 safety, tolerability, pharmacokinetics, and preliminary efficacy in H2 2024.
- Familial Adenomatous Polyposis (FAP) (REC-4881): We have enrolled multiple participants in our TUPELO clinical trial which evaluates REC-4881 in patients with FAP. We are now providing guidance on a data readout and expect to share Phase 2 safety, tolerability, pharmacokinetics, and preliminary efficacy in H1 2025.
- AXIN1 or APC Mutant Cancers (REC-4881): We will evaluate REC-4881 in a Phase 2 biomarker enriched study in patients with unresectable, locally advanced or metastatic cancer with AXIN1 or APC mutations. The IND was accepted by the FDA and we expect to initiate this Phase 2 study in Q4 2023.
- Clostridioides difficile Infection (REC-3964): Our Phase 1 clinical trial is a first-in-human protocol evaluating single and multiple doses of REC-3964 in healthy volunteers and will assess the safety, tolerability and pharmacokinetic profile of REC-3964. Single ascending dose and multiple ascending dose studies are now complete. REC-3964 has been well tolerated and no safety issues have been identified to date. We expect to share Phase 1 safety and pharmacokinetics data in Q3 2023.
- RBM39 HR-Proficient Ovarian Cancer: RBM39 (previously identified as Target Gamma) is a novel CDK12-adjacent target identified by the Recursion OS. We believe we can modulate this target to produce a therapeutic effect in HR-proficient ovarian cancer and potentially in other tumor types. This program is in the preclinical stage and IND-enabling studies are progressing.
- Partnerships
- NVIDIA: In July 2023, we announced a $50 million investment and collaboration with NVIDIA. We will continue to build our own foundation models for biology and chemistry and NVIDIA will assist in optimizing these models, provide priority access to computational resources on NVIDIA’s cloud service DGX Cloud, and potentially host commercially-licensable machine learning and foundation models developed by Recursion on BioNeMo, NVIDIA’s marketplace for generative AI in drug discovery. In this partnership, we will maintain control of our proprietary data and models as well as how and where we could host our technology tools as we expand our business strategy of data as a value driver. Since the announcement in July, we have already deployed our digital chemistry technology together with NVIDIA’s computational resources to predict the ligand-protein interactions for approximately 36 billion compounds in the Enamine REAL Space, reported to be the world’s largest searchable chemical library, where we evaluated 2.8 quadrillion target-compound pairs.
- Roche-Genentech and Bayer: We continue to advance our collaborations to discover potential new therapeutics with our strategic partners Roche-Genentech and Bayer. In the near-term, there is the potential for option exercises associated with partnership programs or option exercises associated with map building initiatives or data sharing.
- Platform
- Digital Chemistry and Generative AI Capabilities: In May 2023, we acquired Cyclica and Valence Discovery to bolster our digital chemistry and generative AI capabilities and drive value across our pipeline, partnerships, and platform. Shortly after closing these acquisitions, we used Cyclica’s digital chemistry tools to predict the protein-ligand interactions for the over 1 million compounds in our internal, non-partnered chemical library. Now, less than one quarter after the closing of these acquisitions, we worked with our partners at NVIDIA to predict the protein-ligand interactions of approximately 36 billion compounds in the Enamine REAL Space, reported to be the world’s largest searchable chemical library.
- Accelerating Pipeline and Partnership Value: For our internal pipeline, we have used our digital chemistry tools to deconvolve proteome-wide biological targets to confirm that certain compounds operate through a novel mechanism of action which was previously predicted by our functional phenomics maps. Such proteomic mapping capabilities provide an additional data layer to efficiently identify the most promising novel chemical series.
- Foundation Model Construction: We continue to use our supercomputer, BioHive-1, to train a proprietary phenomics foundation model. As we have trained on larger quantities of our proprietary data, emergent properties have arisen out of the models and we have seen significant improvements over previous deep learning production models. We are also in the early stages of exploring more powerful and broadly useful foundation models based on our large-scale proprietary multi-omics data, which includes phenomics across 50 human cell types and approximately 1.7 million compounds, multi-timepoint live-cell microscopy, transcriptomics, proteomics, inVivomics, multi-target compound interactions, physicochemical properties, as well predicted protein-ligand relationships. We may explore commercial licensing of some of our models in collaboration with NVIDIA and their BioNeMo platform in the coming year, though our state-of-the-art models will only be available to our team and close partners.
- Large Language Models: One year ago, more than 40 employees were dedicated to exploring our maps of biology and chemistry to initiate programs at Recursion. Today, those same employees have been redeployed and our newest internal programs are being initiated autonomously. This efficiency and scale is through the deployment of large language models to map scientific literature in conjunction with our internally derived proprietary maps to identify opportunities for scientific arbitrage in areas of unmet need. These opportunities are then automatically prioritized for confirmation and validation in our highly-automated wetlabs. This is a significant step towards our vision of autonomous drug discovery and biological exploration.
- Valence Labs - Powered by Recursion: In July 2023 at the International Conference for Machine Learning, we launched Valence Labs, Recursion’s cutting-edge machine learning research center for biology and chemistry in Montréal that aims to promote open-science and academic research. Recursion’s commitment to open-science helps us recruit and retain the best talent in the field of generative AI, allows us to design and set the standards by which ML and AI are deployed in drug discovery, and may drive additional biopharma companies to consider partnering with Recursion to get access to our proprietary state-of-the-art tools, technology, datasets and programs.
Additional Corporate Updates
- Chief Medical Officer: In May 2023, David Mauro, M.D., Ph.D. joined Recursion as its Chief Medical Officer. Dr. Mauro has over 20 years experience in oncology drug development and has guided more than 25 Investigational New Drug candidates through the translational, preliminary, and later stages of development at various companies.
- Chief Legal Officer: In July 2023, Recursion named Nathan Hatfield, J.D., M.B.A. as Chief Legal Officer. Mr. Hatfield has worked at Recursion for over 6 years, previously serving as SVP and Head of Legal. Prior to Recursion, Mr. Hatfield was a securities attorney at the law firm Wilson Sonsini Goodrich & Rosati.
- Toronto Office: In June 2023, we celebrated the opening of our Canadian Headquarters in Toronto with government officials as well as members of the technology and biotechnology communities.
- ESG Reporting: In June 2023, Recursion received a favorable ESG Risk Rating from Morningstar Sustainalytics which ranked Recursion as the #1 biotechnology company out of approximately 400 companies and the #14 pharmaceuticals company out of approximately 900 companies.
Second Quarter 2023 Financial Results
- Cash Position: Cash and cash equivalents were $405.9 million as of June 30, 2023. This cash position does not include the recent $50 million investment from NVIDIA.
- Revenue: Total revenue was $11.0 million for the second quarter of 2023, compared to $7.7 million for the second quarter of 2022. The increase was due to progress made in our Roche-Genentech collaboration.
- Research and Development Expenses: Research and development expenses were $55.1 million for the second quarter of 2023, compared to $38.4 million for the second quarter of 2022. The increase in research and development expenses was due to increased platform costs as we have expanded and upgraded our capabilities.
- General and Administrative Expenses: General and administrative expenses were $28.3 million for the second quarter of 2023, compared to $21.2 million for the second quarter of 2022. The increase in general and administrative expenses was due to an increase in salaries and wages of $3.0 million and increases in software and depreciation expense.
- Net Loss: Net loss was $76.7 million for the second quarter of 2023, compared to a net loss of $65.6 million for the second quarter of 2022.
About Recursion
Recursion is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest proprietary biological and chemical datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale — up to millions of wet lab experiments weekly — and massive computational scale — owning and operating one of the most powerful supercomputers in the world, Recursion is uniting technology, biology and chemistry to advance the future of medicine.
Recursion is headquartered in Salt Lake City, where it is a founding member of BioHive, the Utah life sciences industry collective. Recursion also has offices in Toronto, Montréal and the San Francisco Bay Area. Learn more at www.Recursion.com, or connect on Twitter and LinkedIn.
Media Contact
This email address is being protected from spambots. You need JavaScript enabled to view it.
Investor Contact
This email address is being protected from spambots. You need JavaScript enabled to view it.
Consolidated Statements of Operations
| Recursion Pharmaceuticals, Inc. |
| Condensed Consolidated Statements of Operations (unaudited) |
| (in thousands, except share and per share amounts) |
| |
| | | Three months ended | | Six months ended |
| | | June 30, | | June 30, |
| Revenue | | 2023 | | | 2022 | | | | 2023 | | | 2022 | |
| | Operating revenue | $ | 11,016 | | $ | 7,653 | | | $ | 23,150 | | $ | 12,952 | |
| | Grant revenue | | 1 | | | 21 | | | | 1 | | | 55 | |
| Total revenue | | 11,017 | | | 7,674 | | | | 23,151 | | | 13,007 | |
| | | | | | | |
| Operating costs and expenses | | | | | |
| | Cost of revenue | | 9,382 | | | 14,227 | | | | 21,829 | | | 22,026 | |
| | Research and development | | 55,060 | | | 38,439 | | | | 101,737 | | | 70,880 | |
| | General and administrative | | 28,290 | | | 21,199 | | | | 51,165 | | | 42,273 | |
| Total operating costs and expenses | | 92,732 | | | 73,865 | | | | 174,731 | | | 135,179 | |
| | | | | | | |
| Loss from operations | | (81,715 | ) | | (66,191 | ) | | | (151,580 | ) | | (122,172 | ) |
| | Other income, net | | 4,989 | | | 631 | | | | 9,527 | | | 633 | |
| Net loss | $ | (76,726 | ) | $ | (65,560 | ) | | $ | (142,053 | ) | $ | (121,539 | ) |
| | | | | | | |
| Per share data | | | | | |
| Net loss per share of Class A, B and Exchangeable common stock, basic and diluted | $ | (0.38 | ) | $ | (0.38 | ) | | $ | (0.71 | ) | $ | (0.71 | ) |
| Weighted-average shares (Class A, B and Exchangeable) outstanding, basic and diluted | | 201,415,475 | | | 172,212,390 | | | | 198,957,804 | | | 171,455,595 | |
| | | | | | | |
Consolidated Balance Sheets
| Recursion Pharmaceuticals, Inc. |
| Condensed Consolidated Balance Sheets (unaudited) |
| (in thousands) |
| | | | |
| | | June 30, | December 31, |
| | | | 2023 | | | 2022 | |
| Assets | | |
| | Current assets | | |
| | Cash and cash equivalents | $ | 405,870 | | $ | 549,912 | |
| | Restricted cash | | 3,325 | | | 1,280 | |
| | Other receivables | | 3,051 | | | 2,753 | |
| | Other current assets | | 18,774 | | | 15,869 | |
| | Total current assets | | 431,020 | | | 569,814 | |
| | | | |
| | Restricted cash, non-current | | 7,629 | | | 7,920 | |
| | Property and equipment, net | | 89,768 | | | 88,192 | |
| | Operating lease right-of-use assets | | 34,899 | | | 33,255 | |
| | Intangible assets, net | | 42,757 | | | 1,306 | |
| | Goodwill | | 60,516 | | | 801 | |
| | Other assets, non-current | | 110 | | | - | |
| | Total assets | $ | 666,699 | | $ | 701,288 | |
| | | | |
| Liabilities and stockholders’ equity | | |
| | Current liabilities | | |
| | Accounts payable | $ | 2,086 | | $ | 4,586 | |
| | Accrued expenses and other liabilities | | 32,873 | | | 32,904 | |
| | Unearned revenue | | 73,105 | | | 56,726 | |
| | Notes payable | | 676 | | | 97 | |
| | Operating lease liabilities | | 5,219 | | | 5,952 | |
| | Total current liabilities | | 113,959 | | | 100,265 | |
| | | | |
| | Unearned revenue, non-current | | 32,436 | | | 70,261 | |
| | Notes payable, non-current | | 1,155 | | | 536 | |
| | Operating lease liabilities, non-current | | 45,850 | | | 44,420 | |
| | Deferred tax liabilities | | 4,336 | | | - | |
| | Total liabilities | | 197,736 | | | 215,482 | |
| | | | |
| | Commitments and contingencies | | |
| | | | |
| | Stockholders’ equity | | |
| | Common stock (Class A, B and Exchangeable) | | 2 | | | 2 | |
| | Additional paid-in capital | | 1,250,570 | | | 1,125,360 | |
| | Accumulated deficit | | (781,609 | ) | | (639,556 | ) |
| | Total stockholder's equity | | 468,963 | | | 485,806 | |
| | | | |
| | Total liabilities and stockholders’ equity | $ | 666,699 | | $ | 701,288 | |
| | | | |
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements'' within the meaning of the Securities Litigation Reform Act of 1995, including, without limitation, those regarding the outcomes and benefits expected from the NVIDIA partnership and Cyclica and Valence Discovery acquisitions and the launch of Valence Labs; early and late stage discovery, preclinical, and clinical programs, including timelines for data readouts; licenses and collaborations, including option exercises by partners and additional partnerships; prospective products and their potential future indications and market opportunities; Recursion OS and other technologies; business and financial plans and performance, including cash runway; and all other statements that are not historical facts. Forward-looking statements may or may not include identifying words such as “plan,” “will,” “expect,” “anticipate,” “intend,” “believe,” “potential,” “could,” “continue,” and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties such as those described under the heading “Risk Factors” in our filings with the U.S. Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q and our Annual Report on Form 10-K. All forward-looking statements are based on management’s current estimates, projections, and assumptions, and Recursion undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.