ACTON, Mass. / Feb 07, 2024 / Business Wire / Insulet Corporation (NASDAQ: PODD) (Insulet or the Company), the global leader in tubeless insulin pump technology with its Omnipod® brand of products, today announced it has received CE mark approval under the European Medical Device Regulation for the added compatibility of the Abbott FreeStyle Libre 2 Plus sensor with Insulet’s Omnipod 5 Automated Insulin Delivery System for individuals aged two years and older with type 1 diabetes.
“The addition of the Abbott FreeStyle Libre 2 Plus sensor to our CGM compatibility expands accessibility of Omnipod 5, which has been a priority for Insulet since the system launched in 2022,” said Patrick Crannell, Senior Vice President and International General Manager of Insulet. “We are excited to have achieved this key milestone, which allows us to bring Omnipod 5 and choice of CGM to more people with diabetes.”
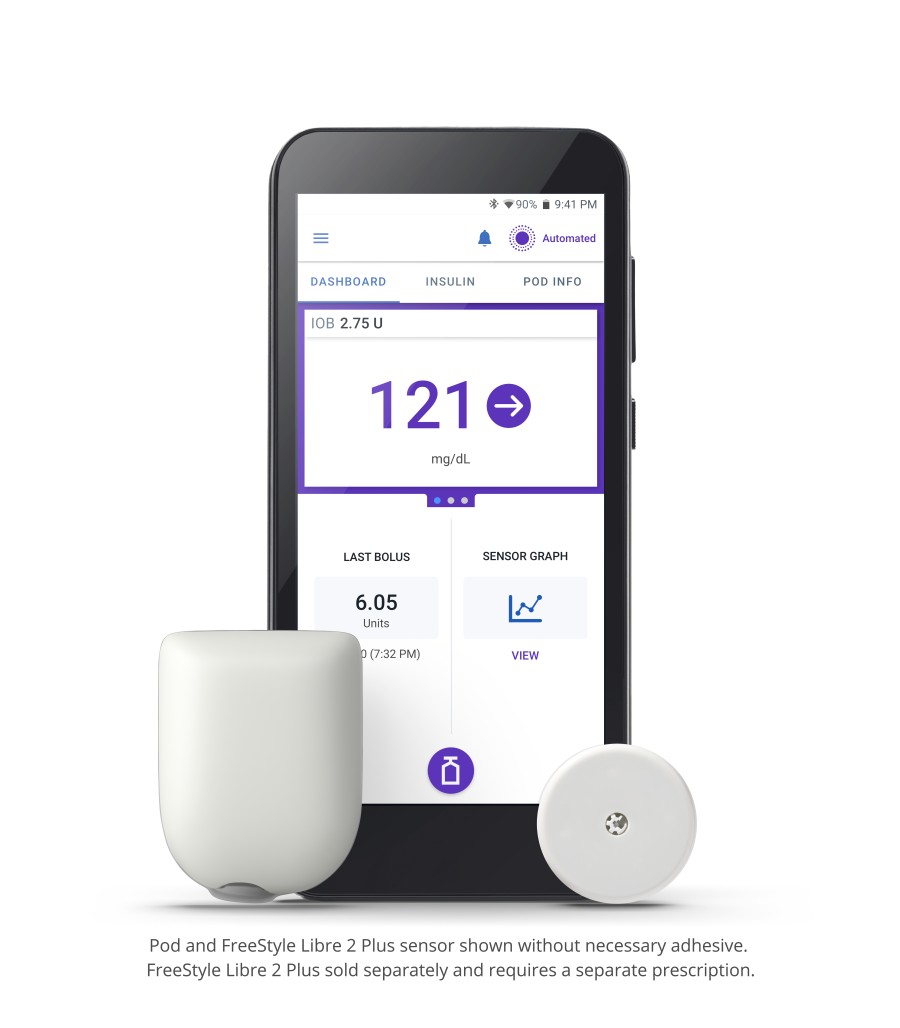
Omnipod 5 is the first and only tubeless hybrid closed loop system (also known as automated insulin delivery) that is approved for CE marking and integrated with two CGM sensor brands, Abbott FreeStyle Libre and Dexcom. The Omnipod 5 System consists of the tubeless Pod enhanced with SmartAdjust™ Technology and the Omnipod 5 Controller with its integrated Smartbolus Calculator.
The Pod’s SmartAdjust technology receives a CGM value and trend and predicts where glucose will be 60 minutes into the future. The System corrects every five minutes based on the user’s desired and customized glucose target.
Insulet expects Omnipod 5 integration with the Abbott FreeStyle Libre 2 Plus sensor to be available first in the United Kingdom and Netherlands in a phased launch in the first half of 2024, with additional markets to follow.
To learn more about Omnipod 5, visit the Omnipod website.
About Insulet Corporation
Insulet Corporation (NASDAQ: PODD), headquartered in Massachusetts, is an innovative medical device company dedicated to simplifying life for people with diabetes and other conditions through its Omnipod product platform. The Omnipod Insulin Management System provides a unique alternative to traditional insulin delivery methods. With its simple, wearable design, the disposable Pod provides up to three days of non-stop insulin delivery, without the need to see or handle a needle. Insulet’s flagship innovation, the Omnipod 5 Automated Insulin Delivery System, is a tubeless automated insulin delivery system, integrated with a continuous glucose monitor to manage blood sugar with no multiple daily injections, zero fingersticks, and can be fully controlled by a compatible personal smartphone or the Omnipod 5 Controller. Insulet also leverages the unique design of its Pod by tailoring its Omnipod technology platform for the delivery of non-insulin subcutaneous drugs across other therapeutic areas. For more information, please visit insulet.com and omnipod.com.
Forward-Looking Statement
This press release may contain forward-looking statements concerning Insulet's expectations, anticipations, intentions, beliefs, or strategies regarding the future. These forward-looking statements are based on its current expectations and beliefs concerning future developments and their potential effects on Insulet. There can be no assurance that future developments affecting Insulet will be those that it has anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond its control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, and other risks and uncertainties described in its Annual Report on Form 10-K, which was filed with the Securities and Exchange Commission on February 24, 2023 in the section entitled "Risk Factors," and in its other filings from time to time with the Securities and Exchange Commission. Should one or more of these risks or uncertainties materialize, or should any of its assumptions prove incorrect, actual results may vary materially from those projected in these forward-looking statements. Insulet undertakes no obligation to publicly update or revise any forward-looking statements.
©2024 Insulet Corporation. Omnipod and SmartAdjust are trademarks or registered trademarks of Insulet Corporation in the United States of America and other various jurisdictions. All rights reserved. The sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott and used with permission. Dexcom is a registered trademark of Dexcom, Inc. and used with permission. All other trademarks are the property of their respective owners.
| Last Trade: | US$266.57 |
| Daily Change: | 10.01 3.90 |
| Daily Volume: | 1,110,586 |
| Market Cap: | US$18.700B |
November 07, 2024 August 26, 2024 August 20, 2024 August 14, 2024 | |

Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB