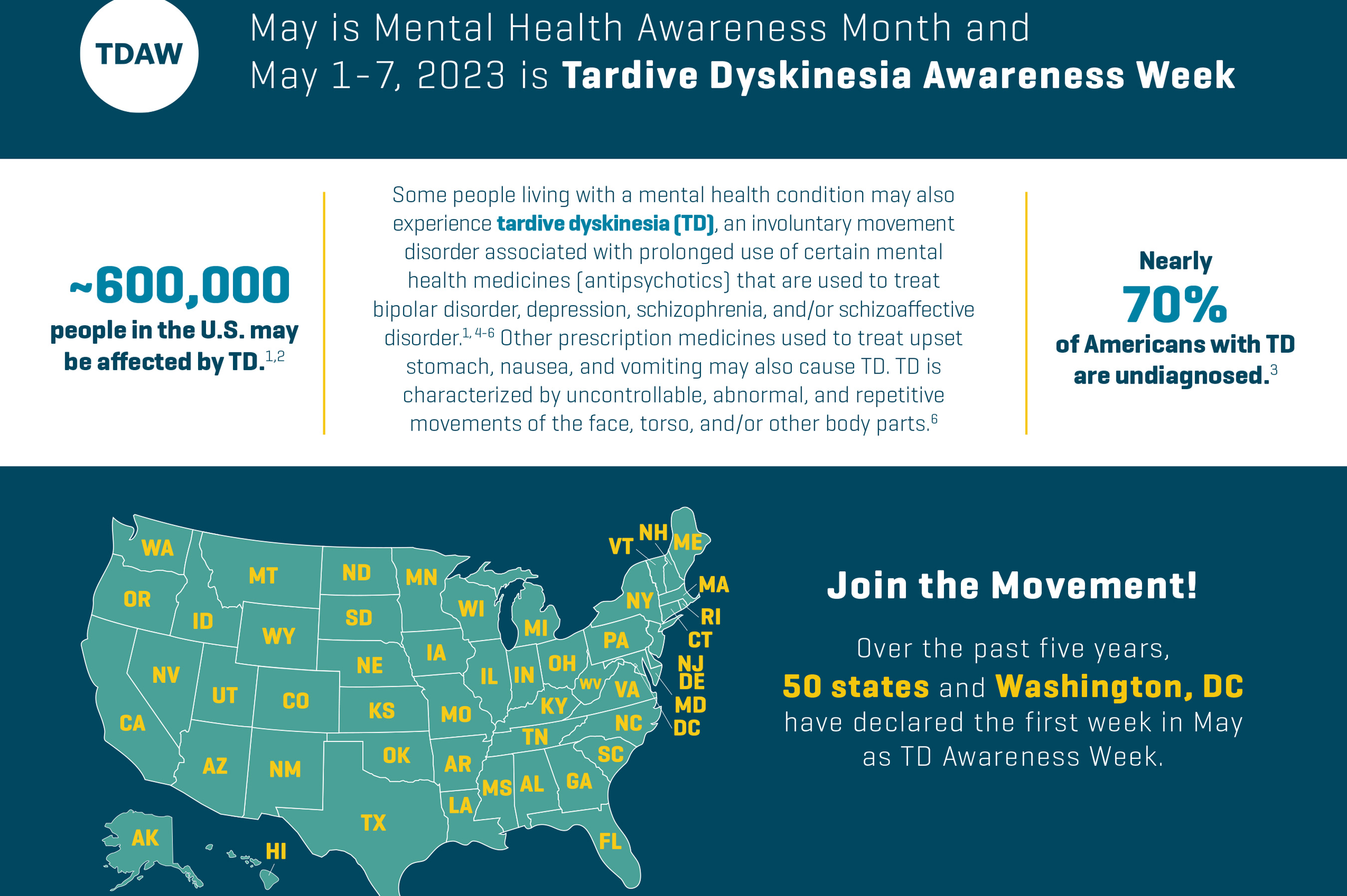
SAN DIEGO, May 1, 2023 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced its continued support of increasing awareness regarding the prevalence, impact and importance of screening for tardive dyskinesia (TD), during Tardive Dyskinesia Awareness Week, May 1-7. TD is an involuntary movement disorder associated with prolonged use of certain mental health medicines (antipsychotics) that can be used to treat bipolar disorder, depression, schizophrenia and schizoaffective disorder.1,3,4
Experience the full interactive Multichannel News Release here: https://www.multivu.com/players/English/9147351-neurocrine-biosciences-tardive-dyskinesia-awareness-week/
This year marks the sixth consecutive year the mental health advocacy community, states across the country and Washington, D.C., have recognized the first week of May as TD Awareness Week, which also occurs during Mental Health Awareness Month. TD Awareness Week aims to recognize and support the approximately 600,000 people impacted by the condition.1,2
"Support for TD Awareness Week continues to grow among legislators and the advocacy community to acknowledge those living with this lesser-known condition that impacts people physically, socially and emotionally," said Josie Cooper, Executive Director of the Movement Disorders Policy Coalition. "It's important that we continue to raise awareness around TD in an effort to help ensure those who are already living with a mental health condition receive a proper diagnosis and the support they need."
TD is a chronic condition that is unlikely to improve without treatment.1 The physical, social and emotional toll of TD can impact one's ability to work, drive, button a shirt, eat or even hold a cup of coffee.2,5,6 TD may also exacerbate feelings of vulnerability, embarrassment and frustration due to uncontrollable movements.5-9 In a survey (n=250), more than half (51 percent) reported that TD had affected their ability to sleep.*† Nearly half (44 percent) stated TD affected their ability to work and leave the house, and more than one-third (34 percent) reported it impacted their ability to eat and drink.*†
"TD Awareness Week is an important time to acknowledge TD and the importance of regular screenings for people taking antipsychotic medication to improve diagnosis rates," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "While we are encouraged that diagnosis rates of TD have increased since the week was first recognized in 2018, there is still more work to be done, as nearly 70 percent of patients living with the condition have not yet been diagnosed."2
It's crucial that people taking antipsychotic medication understand the importance of being monitored for drug-induced movement disorders (DIMDs), such as TD, with regularly scheduled in-person appointments. In a recent survey, 69 percent of TD patients (n=250) experiencing moderate to severe involuntary movement symptoms reported that their mental health had been impacted by their involuntary movements.*§ Screening for TD can help healthcare providers establish a diagnosis and treatment plans for patients, while also following the progression of the movement disorder.10
The 2020 American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia recommends that a physical assessment and visual examination of the body should be conducted at least every six to 12 months for people taking antipsychotic medication who are at risk of TD.10 The Real-World Tele-Health Evaluation of Tardive Dyskinesia Symptoms Communication/Observation Procedure Evaluation in Outpatient Clinical Settings, or TeleSCOPE, study, showed that telehealth visits (either by phone only or with video) led to fewer patients being evaluated, diagnosed and monitored for DIMDs.11
Learn more about TD, living with TD and how to treat TD by visiting TalkAboutTD.com.
About Tardive Dyskinesia Awareness Week
Over the past six years, 50 states, Washington, D.C., and various mental health advocacy organizations have recognized the first week of May (1-7) as Tardive Dyskinesia (TD) Awareness Week. This awareness week recognizes the approximately 600,000 Americans living with this involuntary movement disorder.1,2 Some states and local advocacy groups are also hosting virtual educational briefings to raise awareness. TD Awareness Week encourages the mental health and broader communities to learn about ways to recognize TD symptoms, understand the burden of the condition, and talk to their healthcare provider if they think they or someone they know may have the condition.
As part of Neurocrine Biosciences' commitment to TD education, more information is available on Neurocrine.com/TD-Awareness and resources are available at TalkAboutTD.com. These resources can help patients and care partners understand TD and recognize its symptoms, request support, and have a conversation with their healthcare provider about ways to manage their TD, including treatment options. Healthcare professionals can also visit MIND-TD.com to learn about differential diagnosis of TD and other movement disorders. For more information, follow and join the conversation online by sharing #TDAwarenessWeek.
About Tardive Dyskinesia (TD)
Tardive dyskinesia (TD) is a movement disorder that is characterized by uncontrollable, abnormal and repetitive movements of the face, torso and/or other body parts, which may be disruptive and negatively impact patients. The condition is associated with taking certain kinds of mental health medicines (like antipsychotics) that help control dopamine receptors in the brain. Taking antipsychotics commonly prescribed to treat mental illnesses such as depression, bipolar disorder, schizophrenia and schizoaffective disorder, and certain medications to treat upset stomach, nausea and vomiting (metoclopramide and prochlorperazine), are associated with TD. In patients with TD, these treatments are thought to result in irregular dopamine signaling in a region of the brain that controls movement. The symptoms of TD can be severe and are often persistent and irreversible. TD is estimated to affect approximately 600,000 people in the U.S.
About Neurocrine Biosciences, Inc.
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine, and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, Parkinson's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, Twitter and Facebook. (*in collaboration with AbbVie)
Neurocrine and the Neurocrine logo are registered trademarks of Neurocrine Biosciences, Inc.
REFERENCES:
*Base: Patient ATU 2022. Target patients (diagnosed TD or suspected TD) n=250.
†Responses based on survey questions: Since first experiencing involuntary movements, how has your ability to perform the following daily activities been affected, if at all? Please use a scale of 1 to 5 when 1 means "Not affected at all," and 5 means "Extremely negatively affected." How would you describe the severity of your involuntary movements? Results shown include responses ≥3.
§Responses based on survey questions: Since first experiencing involuntary movements, how have the following areas of your life been affected, if at all. Please use a scale of 1 to 5 when 1 means "Not affected at all," and 5 means "Extremely negatively affected." How would you describe the severity of your involuntary movements? Results shown include responses ≥3.
©2023 Neurocrine Biosciences, Inc. All Rights Reserved. CP-TD-US-1323 04/2023

| Last Trade: | US$135.42 |
| Daily Change: | 0.46 0.34 |
| Daily Volume: | 1,924,196 |
| Market Cap: | US$13.710B |
December 20, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB