SAN DIEGO, Nov. 2, 2023 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced interim results from the ongoing open-label KINECT®-HD2 study about INGREZZA® (valbenazine) capsules when used for the long-term treatment of adults with chorea associated with Huntington's disease (HD). Interim data suggest one-capsule, once-daily INGREZZA improved chorea at the first evaluation at Week 2 with sustained efficacy through Week 50. These data will be presented at the 30th Annual Meeting of the Huntington Study Group on November 2–4 in Phoenix.
"These interim data provide insight on the clinically meaningful and sustained improvements participants are experiencing with INGREZZA for the treatment of chorea," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences®. "We look forward to analyzing additional data as they become available."
KINECT-HD2 includes adults with genetically confirmed motor-manifest HD (n=127), most of whom (n=98) completed KINECT-HD, a Phase 3, randomized, double-blind, placebo-controlled study. Both studies were conducted in collaboration with the Huntington Study Group (HSG) and contributed to the recent U.S. Food and Drug Administration approval of INGREZZA for chorea associated with HD. Concomitant antipsychotic use is also being explored in the open-label study.
The current interim results from KINECT-HD2 (Sustained Improvements With Once-Daily Valbenazine in Chorea Associated With Huntington's Disease: Interim Results From a Long-Term Open-Label Study, Poster # 64) suggest that INGREZZA improved chorea at the first evaluation (Week 2) when participants were taking the lowest dose of 40 mg, with efficacy sustained through Week 50 at ≤ 80 mg (Figure 1).
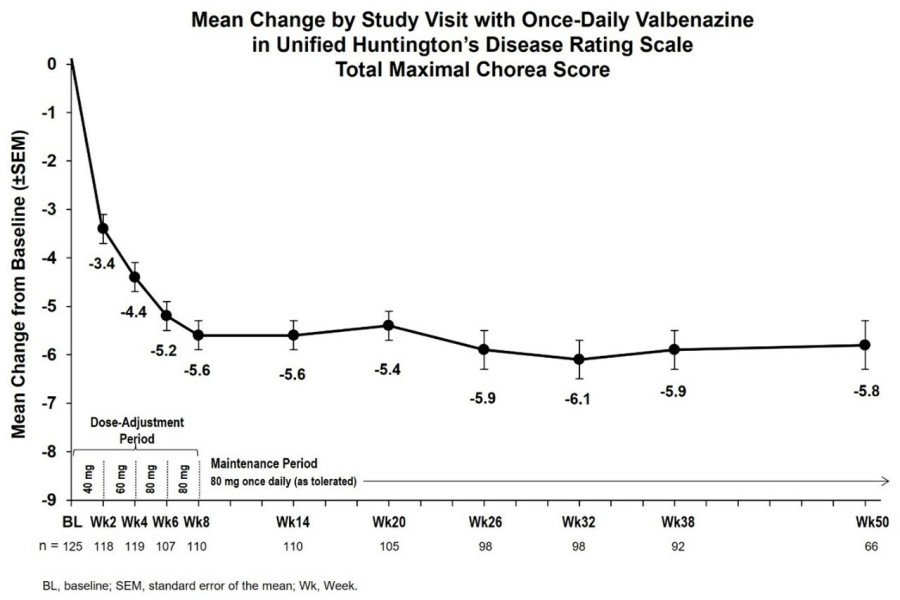
More than half of participants (60.9 percent) and investigators (58.9 percent) rated symptoms as "much improved" or "very much improved" at Week 6, and about three-quarters of participants (74.2 percent) and investigators (76.9 percent) rated symptoms as "much improved" or "very much improved" by Week 50. The most common treatment-emergent adverse events at the time of the analysis were consistent with those observed in KINECT-HD, including falls (30.4 percent), fatigue (24.0 percent) and somnolence (24.0 percent).
Neurocrine Biosciences will also present new data from KINECT-HD (A Wearable Movement Sensor Substudy of KINECT-HD, a Phase 3 Trial of Valbenazine for the Treatment of Chorea Associated With Huntington's Disease, Poster #65), which was the first Phase 3 clinical trial to include a wearable movement sensor substudy. Significant improvements in truncal chorea and gait asymmetry measures were seen from baseline to maintenance following the Week 10 visit in the INGREZZA-treated group (P < 0.05) compared to placebo. These findings support how digital measurements can be used to detect meaningful symptom changes in individuals with HD to improve clinical research and care.
Additional HD chorea presentations at the 30th Annual Meeting of the HSG include:
The full abstracts can be accessed on the Journal of Huntington's Disease website.
About Chorea Associated with Huntington's Disease (HD)
Huntington's disease (HD) is a hereditary progressive neurodegenerative disorder in which the loss of certain neurons within the brain causes motor, cognitive and psychiatric symptoms. Symptoms generally appear between the ages of 30 and 50 years and worsen over a 10- to 25-year period. Most people with HD experience chorea, an abnormal involuntary movement disorder, characterized by irregular and unpredictable movements. Chorea can affect various body parts and interfere with motor coordination, gait, swallowing and speech. HD is estimated to affect approximately 41,000 adults in the U.S., with more than 200,000 at risk of inheriting the disease.
About KINECT®-HD
KINECT®-HD was a Phase 3, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy of valbenazine as a once-daily treatment to reduce chorea associated with Huntington's disease (HD) and evaluate the safety and tolerability of valbenazine in patients with HD. The study enrolled 128 adults 18 to 75 years of age who were diagnosed with motor-manifest HD and who had sufficient chorea symptoms to meet study protocol criteria.
KINECT-HD used the Unified Huntington's Disease Rating Scale (UHDRS®) Total Maximal Chorea (TMC) score as the primary efficacy endpoint. The secondary endpoints included Clinical Global Impression of Change (CGI-C) response status and Patient Global Impression of Change (PGI-C) response status for valbenazine treatment. Treatment with valbenazine resulted in a placebo-adjusted mean reduction in the TMC score of 3.2 units (P < 0.0001), indicating a substantial improvement in chorea. Secondary endpoints of CGI-C response status and PGI-C response status were also statistically significant and supported the improvements in TMC score that were seen over the 12-week study period.
Treatment-emergent adverse events in this study were generally consistent with the known safety profile of valbenazine. The most common adverse reactions in patients with HD included somnolence and sedation, urticaria, rash and insomnia.
View the complete study results from the Phase 3 KINECT-HD study published in The Lancet Neurology online edition. For more information on the KINECT-HD study, please visit HuntingtonStudyGroup.org.
About KINECT®-HD2
KINECT®-HD2 is an ongoing open-label study to evaluate the long-term safety and tolerability, as well as the maintenance of effects, of INGREZZA in patients with chorea associated with Huntington's disease (HD). The 156-week study has enrolled more than 150 adults 18 to 75 years of age who have been diagnosed with motor-manifest HD and who have sufficient chorea symptoms to meet study protocol criteria. Concomitant antipsychotic use is allowed in the study. For more information on the KINECT-HD2 study, please visit HuntingtonStudyGroup.org or ClinicalTrials.gov.
About Huntington Study Group / HSG Clinical Research, Inc.
The Huntington Study Group (HSG), a not-for-profit organization founded in 1993 in Rochester, NY, and its wholly owned subsidiary, HSG Clinical Research, Inc., designs and conducts clinical trials through the world's first and largest collaborative network with thousands of members at more than 130 HSG credentialed research sites worldwide. HSG collaborated with the respective study sponsors to complete the three pivotal clinical trials that led to the only FDA-approved medications for Huntington's disease associated chorea. The organization is dedicated to improving the lives of people impacted by Huntington's disease through research, education, and collaboration. For more information, visit www.huntingtonstudygroup.org.
About INGREZZA® (valbenazine) Capsules
INGREZZA is the only one-capsule, once-daily selective vesicular monoamine transporter 2 (VMAT2) inhibitor approved by the U.S. Food and Drug Administration for the treatment of adults with tardive dyskinesia and the treatment of chorea associated with Huntington's disease (HD).
INGREZZA, developed by Neurocrine Biosciences, selectively inhibits VMAT2 with no appreciable binding affinity for VMAT1, dopaminergic (including D2), serotonergic, adrenergic, histaminergic or muscarinic receptors. While the specific way INGREZZA works to treat TD and HD chorea is not fully understood, INGREZZA selectively targets VMAT2 to inhibit the release of dopamine, a chemical in the brain that helps control movement. INGREZZA is believed to reduce extra dopamine signaling, which may lead to fewer uncontrollable movements. Additionally, INGREZZA can be taken as one-capsule once-daily, together with most psychiatric medications such as antipsychotics or antidepressants. INGREZZA dosages approved for use are 40 mg, 60 mg and 80 mg capsules. INGREZZA is not approved in any other dosage form.
Important Information
Approved Uses
INGREZZA® (valbenazine) capsules is a prescription medicine used to treat adults with:
It is not known if INGREZZA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
VMAT2 inhibitors, including INGREZZA, can cause serious side effects in people with Huntington's disease, including: depression, suicidal thoughts, or suicidal actions. Tell your healthcare provider before you start taking INGREZZA if you have Huntington's disease and are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is especially important when INGREZZA is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of hurting yourself.
Do not take INGREZZA if you:
INGREZZA may cause serious side effects, including:
Before taking INGREZZA, tell your healthcare provider about all of your medical conditions including if you: have liver or heart problems, are pregnant or plan to become pregnant, or are breastfeeding or plan to breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Sleepiness (sedation) is a common side effect with INGREZZA. While taking INGREZZA, do not drive a car or operate dangerous machinery until you know how INGREZZA affects you. Drinking alcohol and taking other drugs that may also cause sleepiness while you are taking INGREZZA may increase any sleepiness caused by INGREZZA.
The most common side effect of INGREZZA in people with tardive dyskinesia is sleepiness (somnolence).
The most common side effects of INGREZZA in people with Huntington's disease are sleepiness (somnolence), allergic itching, rash, and trouble getting to sleep or staying asleep.
These are not all of the possible side effects of INGREZZA. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see INGREZZA full Prescribing Information, including Boxed Warning.
About Neurocrine Biosciences
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine, and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, Parkinson's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit Neurocrine.com, and follow the company on LinkedIn, X (formerly Twitter) and Facebook. (*in collaboration with AbbVie)
NEUROCRINE, NEUROCRINE BIOSCIENCES, the Neurocrine logo, INGREZZA, the INGREZZA logo and KINECT are registered trademarks of Neurocrine Biosciences, Inc.
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the potential benefits to be derived from INGREZZA for the treatment of chorea associated with Huntington's disease (HD), and the value INGREZZA for the treatment of chorea associated with HD brings to patients. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include: risks and uncertainties associated with Neurocrine Biosciences' business and finances in general, as well as risks and uncertainties associated with the commercialization of INGREZZA for the treatment of chorea associated with HD; whether INGREZZA for the treatment of chorea associated with HD receives adequate reimbursement from third-party payors; the degree and pace of market uptake of INGREZZA for the treatment of chorea associated with HD; risks and uncertainties relating to competitive products and technological changes that may limit demand for INGREZZA for the treatment of chorea associated with HD; risks associated with the Company's dependence on third parties for development and manufacturing activities related to INGREZZA for the treatment of chorea associated with HD, and the ability of the Company to manage these third parties; risks that additional regulatory submissions for INGREZZA for the treatment of chorea associated with HD or other product candidates may not occur or be submitted in a timely manner; risks that the FDA or other regulatory authorities may make adverse decisions regarding INGREZZA for the treatment of chorea associated with HD; risks that post-approval INGREZZA for the treatment of chorea associated with HD commitments or requirements may be delayed; risks that INGREZZA for the treatment of chorea associated with HD may be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; risks and uncertainties relating to competitive products and technological changes that may limit demand for INGREZZA for the treatment of chorea associated with HD; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2023. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof.
©2023 Neurocrine Biosciences, Inc. All Rights Reserved.

| Last Trade: | US$138.41 |
| Daily Change: | -1.03 -0.74 |
| Daily Volume: | 262,664 |
| Market Cap: | US$14.010B |
December 20, 2024 | |

Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MORE
Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB