ATLANTA / May 09, 2024 / Business Wire / Researchers from Mind Medicine (MindMed) Inc. (NASDAQ: MNMD) (the “Company” or “MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today presented new studies highlighting the significant impact of GAD in the US at ISPOR 2024, the leading global conference for health economics and outcomes research (HEOR) being held this week in Atlanta.
Three studies examined the impact of GAD on healthcare utilization and costs, workplace productivity, and healthcare quality of life (HC QOL). All three studies demonstrated that the economic and societal impact of GAD is substantial, gets worse when anxiety symptoms are more severe, and is more significant when GAD is present but undiagnosed.
“These studies clearly demonstrate that the burden of GAD on the US healthcare system, employers, and people living with GAD is enormous. We continue to discover evidence that the impact of GAD has been underappreciated and that people living with this disorder are underserved,” said Daniel R. Karlin, MD, MA, Chief Medical Officer of MindMed and senior author of these studies. “We undertook these studies as part of our commitment to highlight the scale of the anxiety crisis and our mission to address this burden. The results of these in-depth analyses speak for themselves.”
The studies were conducted using data from the 2022 National Health and Wellness Survey (NHWS; N=75,261). The NHWS is an annual internet-based survey; all data are self-reported. Recruitment is designed to represent the general US adult population in terms of age, race, ethnicity and gender distribution. Participants were requested to use the clinically validated GAD-7 screening tool as recently recommended by the US Preventive Services Task Force (USPSTF).
Three studies were presented at ISPOR 24:
- Work Productivity and Activity Impairment Associated with Generalized Anxiety Disorder among Adults in the United States
- Health Care Resource Use Associated with Generalized Anxiety Disorder among Adults in the United States
- Health-related Quality of Life Associated with Generalized Anxiety Disorder among Adults in the United States
Key Findings from MindMed HEOR Studies
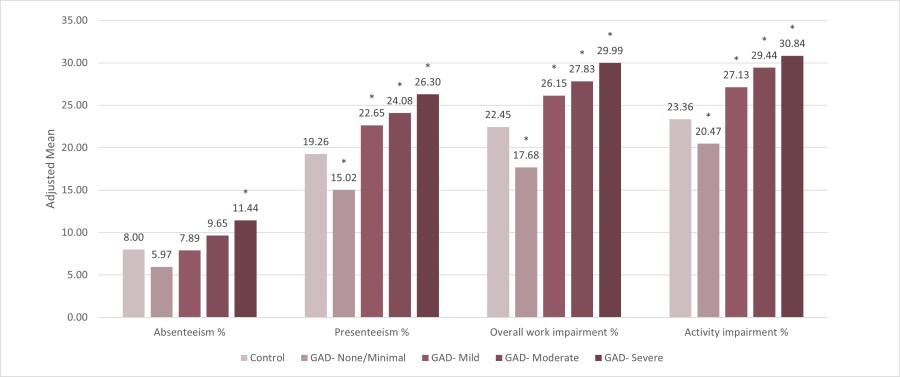
Work Productivity (see Figure 1)
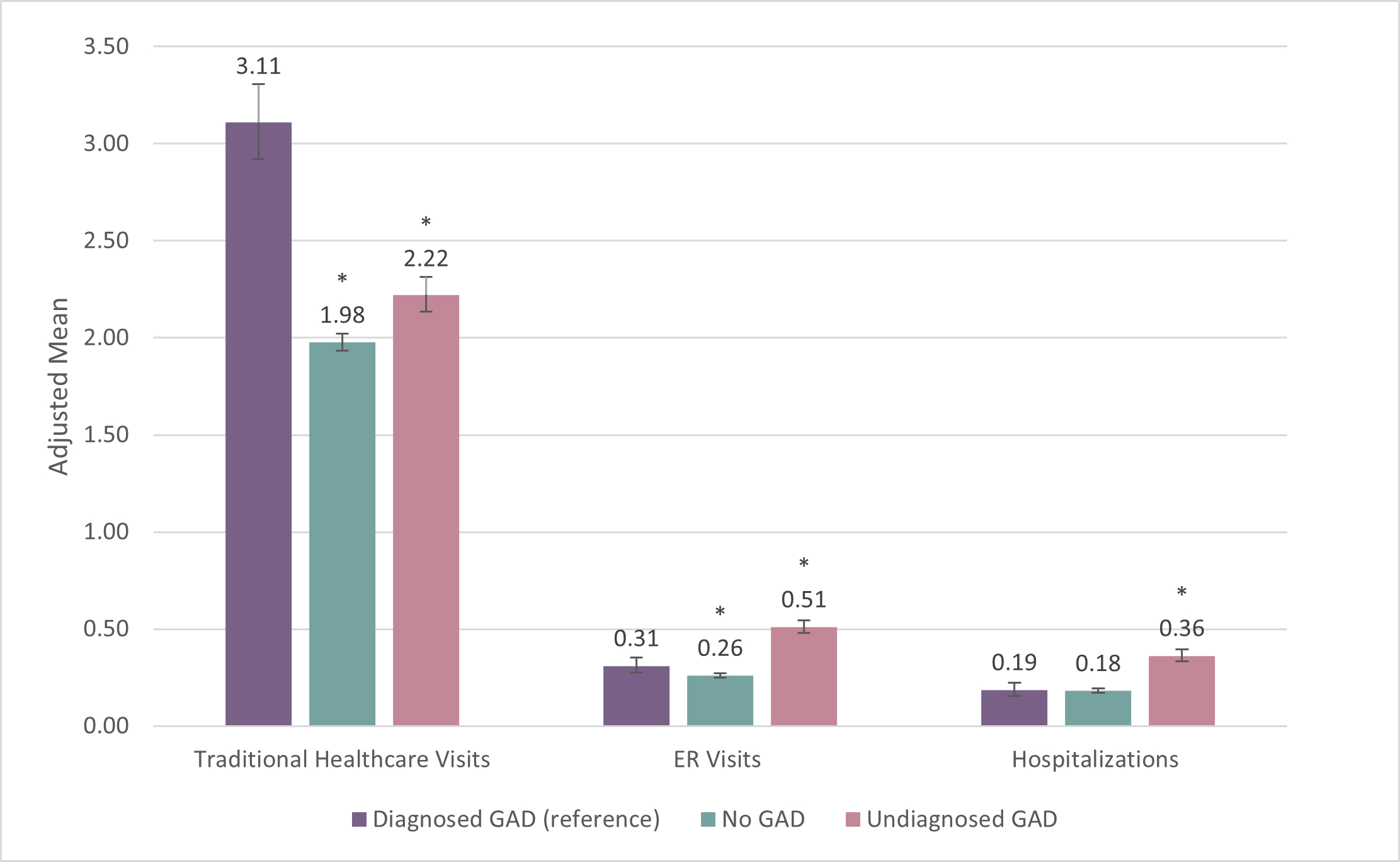
Healthcare Resource Use (HCRU) (see Figure 2)
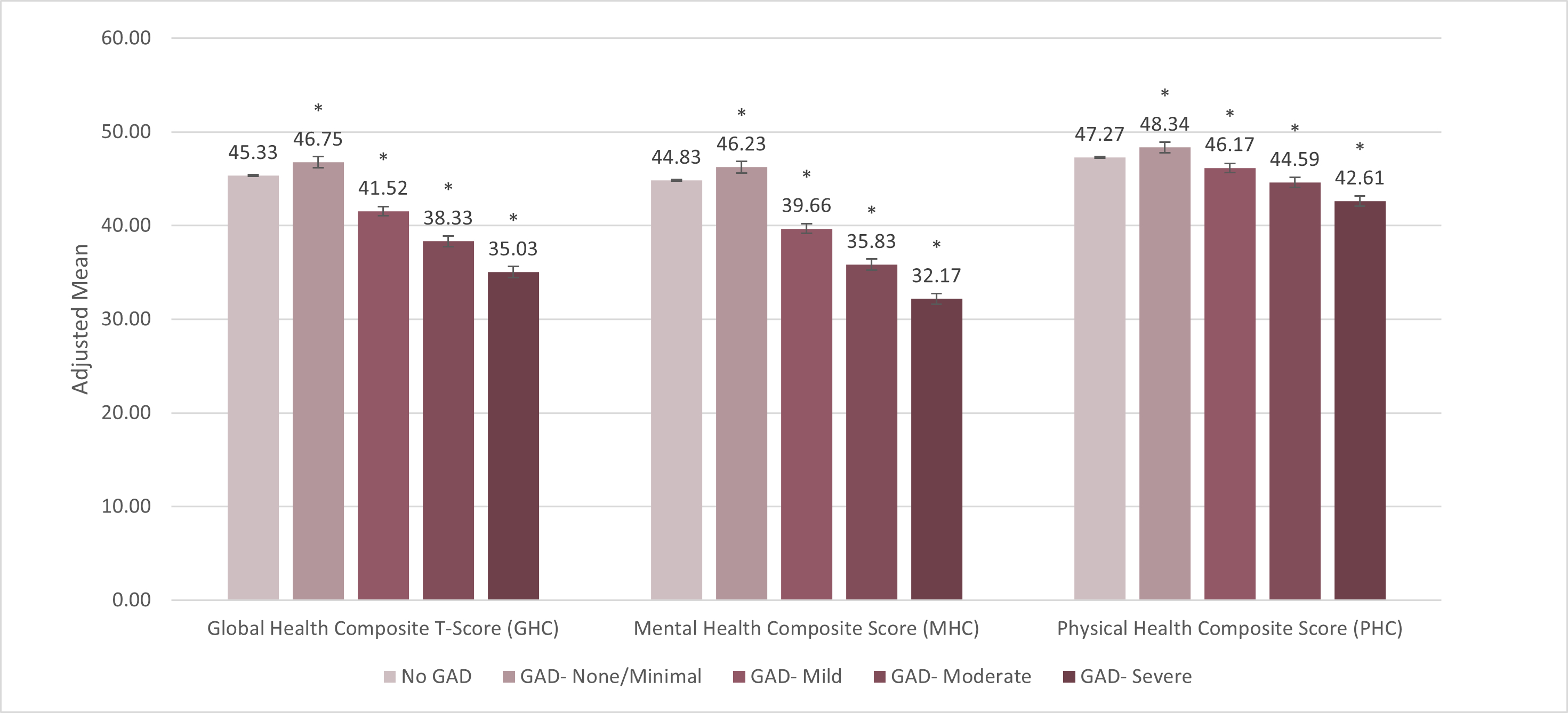
Health-Related Quality of Life (HR QOL) (see Figure 3)
“From my perspective, one key takeaway from our research is the significant and increasing impact of GAD on work productivity, which should concern employers and payers,” according to the lead author of the studies, Phong Duong, PharmD, Vice President of Global Market Access, Health Economics and Outcomes Research at MindMed. “The other key finding was that people with undiagnosed GAD may actually cost more to manage in terms of overall healthcare resource utilization than those who have been diagnosed, which strongly supports screening the general population for GAD using the clinically validated GAD-7 screening tool as recently recommended by the US Preventive Services Task Force (USPSTF).”
About Generalized Anxiety Disorder (GAD)
GAD is a common condition associated with significant impairment that adversely affects millions of people. GAD results in fear, persistent anxiety, and a constant feeling of being overwhelmed. It is characterized by excessive, persistent, and unrealistic worry about everyday things. Approximately 10% of U.S. adults, representing around 20 million people, currently suffer from GAD. This underdiagnosed and underserved indication is associated with significant impairment, less accomplishment at work and reduced labor force participation. Despite the significant personal and societal burden of GAD, there has been little innovation in the treatment of GAD in the past several decades, with the last new drug approval occurring in 2007.
About MindMed
MindMed is a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders. Our mission is to be the global leader in the development and delivery of treatments that unlock new opportunities to improve patient outcomes. We are developing a pipeline of innovative product candidates, with and without acute perceptual effects, targeting neurotransmitter pathways that play key roles in brain health disorders.
MindMed trades on NASDAQ under the symbol MNMD.
Forward-Looking Statements
Certain statements in this news release related to the Company constitute “forward-looking information” within the meaning of applicable securities laws and are prospective in nature. Forward-looking information is not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “will”, “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe”, “potential” or “continue”, or the negative thereof or similar variations. Forward-looking information in this news release includes, but is not limited to, statements regarding the prevalence of undiagnosed GAD patients; the cost of treating GAD patients; the potential impact of GAD on productivity; and the potential benefits of the Company’s product candidates. There can be no guarantees regarding the results of the potential Phase 3 clinical trial or that, following any such trial, MM120 will receive the necessary regulatory approvals. There are numerous risks and uncertainties that could cause actual results and the Company’s plans and objectives to differ materially from those expressed in the forward-looking information, including history of negative cash flows; limited operating history; incurrence of future losses; availability of additional capital; lack of product revenue; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to herein and the risks described in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, under headings such as “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and other filings and furnishings made by the Company with the securities regulatory authorities in all provinces and territories of Canada which are available under the Company’s profile on SEDAR+ at www.sedarplus.ca and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Except as required by law, the Company undertakes no duty or obligation to update any forward-looking statements contained in this release as a result of new information, future events, changes in expectations or otherwise.
| Last Trade: | US$7.83 |
| Daily Change: | 0.35 4.68 |
| Daily Volume: | 328,371 |
| Market Cap: | US$574.170M |
December 16, 2024 December 11, 2024 | |

C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MORE
Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB