HOUSTON, June 14, 2024 /PRNewswire/ -- Moleculin Biotech, Inc., (Nasdaq: MBRX) ("Moleculin" or the "Company"), a clinical stage pharmaceutical company with a broad portfolio of drug candidates targeting hard-to-treat tumors and viruses, today reported additional efficacy findings from the Company's ongoing Phase 1B/2 (MB-106) clinical trial evaluating Annamycin in combination with Cytarabine (also known as "Ara-C" and for which the combination of Annamycin and Ara-C is referred to as AnnAraC) for the treatment of subjects with acute myeloid leukemia (AML). The preliminary data was presented at the European Hematology Association (EHA) 2024 Hybrid Congress. The Company also hosted a data presentation to leading AML experts as part of a KOL meeting held in conjunction with EHA 2024 Congress.
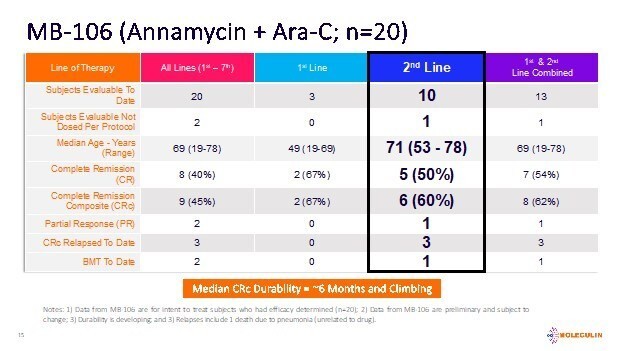
To date, a total of 22 subjects have been enrolled (the Intent-to-Treat population, ITT), 20 (Lines 1st-7th) of whom have completed efficacy evaluations with 9 subjects (45%) achieving a composite complete remission (CRc or CR/CRi), consisting of 8 (40%) subjects with complete remission (CR) and one subject with complete remission with an incomplete recovery of peripheral blood counts (CRi), following treatment with AnnAraC. Efficacy outcomes for 2 additional subjects (enrolled and treated) are pending.
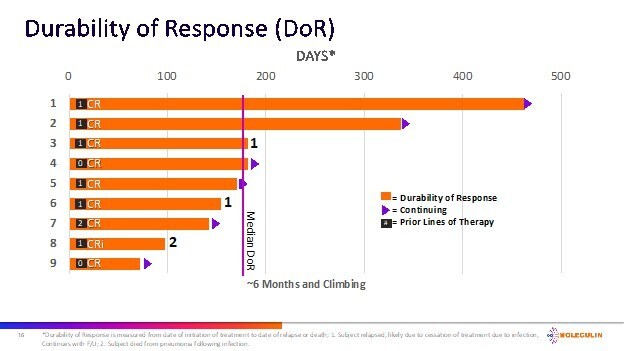
Of the 10 ITT subjects for whom AnnAraC was administered in the 2nd line setting, 5 achieved a CR (50%) and 6 achieved a CRc (60%). Of the 13 subjects in the ITT evaluable population that were 1st or 2nd line treatment, 7 achieved a CR (54%) and 8 achieved a CRc (62%). The mDOR for the 9 subjects who achieved a CRc is approximately 6 months and climbing. Additionally, the median overall survival in the 2nd line subjects (n=10) is approximately 6 months and increasing.
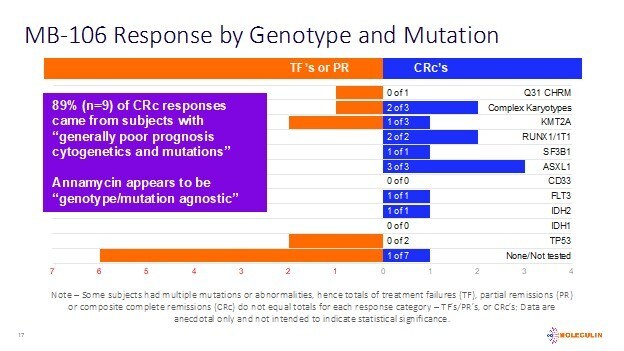
Additionally, 89% of the subjects included in the CRc group (n=9) had cytogenetics and/or mutations generally considered to contribute to a poor prognosis. These include FLT3, IDH2, ASXL1, KMT2A and others. While not yet statistically relevant, the Company believes such cytogenetic and mutation data are informative to clinicians.
"We continue to be highly encouraged by the positive growing body of preliminary clinical data demonstrated by Annamycin in the treatment of patients with AML," commented Walter Klemp, Chairman and Chief Executive Officer of Moleculin. "While still preliminary, we believe the efficacy to date including the climbing durability of response demonstrated by AnnAraC in 2nd line patients continues to significantly exceed the performance reported by any drug currently approved for use in 2nd line AML. We are incredibly pleased with the progress of the trial and the data and continue to advance our preparations for an End of Phase 2 meeting with FDA."
"There remains a significant unmet need for safe and effective therapies for R/R AML. These data are exciting, and I believe further underscore the potential of Annamycin to provide patients and physicians with a promising treatment option in AML," concluded Mr. Klemp.
Currently, the median age of subjects in MB-106 is 69 years. Not including the two most recent subjects, a total of 17 subjects had relapsed/refractory AML and 3 subjects were first line treatment. Two subjects discontinued early due to allergic reactions. All subjects who completed treatment had undergone post-therapy bone marrow assessment (Day 15 or later). No clinically significant signs of cardiotoxicity were noted during or after treatment in any of the subjects enrolled. The combination was well tolerated with myelosuppression and infections being the main adverse events (AEs). All data from MB-106 is preliminary and subject to change.
Data presented at the European Hematology Association (EHA) 2024 Hybrid Congress:
As previously announced, the poster titled "LIPOSOMAL ANNAMYCIN (L-ANN) IN COMBINATION WITH CYTARABINE FOR TREATMENT OF PATIENTS WITH ACUTE MYELOID LEUKAEMIA (AML) REFRACTORY TO OR RELAPSED (R/R) AFTER INDUCTION THERAPY (MB-106 STUDY)," was presented as part of the "Acute myeloid leukemia – Clinical" session by Wolfram C. M. Dempke, MD, PhD, MBA, European Chief Medical Officer of Moleculin. The poster presentation mentioned above will be posted on the Company's website and filed on Form 8-K with the Securities and Exchange Commission.
Annamycin currently has Fast Track Status and Orphan Drug Designation from the U.S. Food and Drug Administration for the treatment of relapsed or refractory acute myeloid leukemia, in addition to Orphan Drug Designation for the treatment of soft tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation for the treatment of relapsed or refractory acute myeloid leukemia from the European Medicines Agency (EMA). For more information about the ongoing MB-106 Phase 1B/2 trial, visit clinicaltrialsregister.eu and reference EudraCT 2020-005493-10 or clinicaltrials.gov and reference NCT05319587.
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical company with a growing pipeline, including Phase 2 clinical programs, for hard-to-treat tumors and viruses. The Company's lead program, Annamycin is a next-generation anthracycline designed to avoid multidrug resistance mechanisms and to eliminate the cardiotoxicity common with currently prescribed anthracyclines. Annamycin is currently in development for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases. All interim and preliminary data related to its active clinical trials are subject to change.
Additionally, the Company is developing WP1066, an Immune/Transcription Modulator capable of inhibiting p-STAT3 and other oncogenic transcription factors while also stimulating a natural immune response, targeting brain tumors, pancreatic and other cancers. Moleculin is also engaged in the development of a portfolio of antimetabolites, including WP1122 for the potential treatment of viruses, as well as certain cancer indications.
For more information about the Company, please visit www.moleculin.com and connect on Twitter, LinkedIn and Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Although Moleculin believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Moleculin has attempted to identify forward-looking statements by terminology including 'believes,' 'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,' 'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,' 'approximately' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission (SEC) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$2.65 |
| Daily Change: | -0.08 -2.93 |
| Daily Volume: | 33,991 |
| Market Cap: | US$7.530M |
November 18, 2024 November 14, 2024 November 11, 2024 October 07, 2024 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB