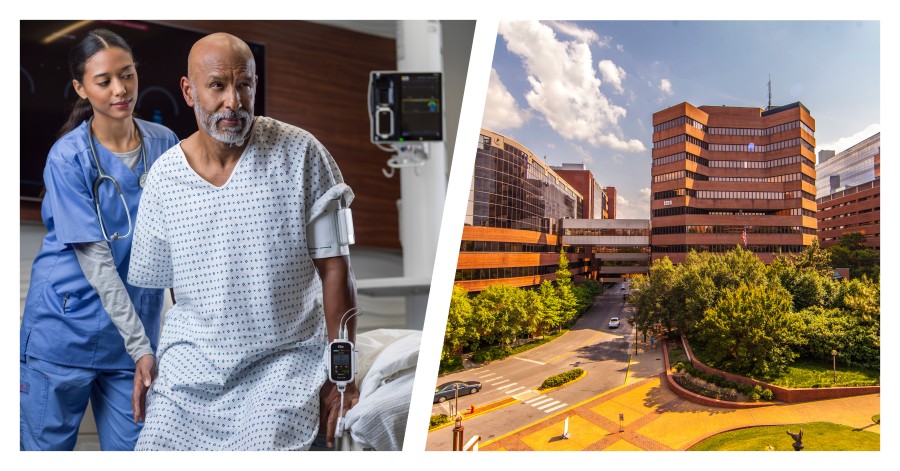
IRVINE, Calif. / Oct 29, 2024 / Business Wire / Masimo (NASDAQ: MASI) today announced that Vanderbilt University Medical Center (VUMC), a renowned healthcare facility in Nashville, Tennessee, is piloting the use of the Masimo Radius VSM™ patient-worn vital signs monitor with Masimo Patient SafetyNet™ supplemental remote monitoring in the Emergency Department (ED) and nontraditional care spaces. Launched as part of a successful pilot program aimed at tackling the ongoing crisis of emergency room congestion, Radius VSM has been used on hallway beds, in the emergency medical service offload area, and on patients in the waiting room who are typically only monitored periodically – thus providing continuous, wireless monitoring for those who may otherwise be left vulnerable to unexpected deterioration.
Radius VSM combines the reliability and accuracy of a bedside monitor with the comfort and freedom of a wearable device. With its implementation alongside Patient SafetyNet, clinicians at VUMC are able to remotely monitor vital signs in real time from centralized view stations, simplifying patient data management, enabling quicker intervention during possible deterioration, and enhancing patient safety—even while a patient is up and moving. The modular, scalable monitoring platform offers a range of physiological measurements, including Masimo SET® pulse oximetry, measure-on-inflation noninvasive blood pressure, continuous temperature, respiration rate, and 3-leadwire electrocardiography (ECG).
By monitoring ED patients with Radius VSM, VUMC is transforming spaces that were traditionally devoid of continuous monitoring into areas of proactive patient care. This level of visibility may help clinicians reduce the use of telemetry, potentially saving time and resources and improving patient throughput and prioritization to other parts of the hospital, such as the general ward or medical and surgical wards. Additionally, Radius VSM’s innovative approach not only enhances the patient and clinician experience but exemplifies how cutting-edge technology can be seamlessly integrated into high-pressure settings like the ED to help streamline continuity of care.
The initial success of VUMC’s pilot program is paving the way for an expanded rollout within the ED designed to elevate care for vulnerable patients. Moreover, the promising results may lead to adoption in other areas of the hospital, such as medical and surgical wards, broadening the impact of Masimo’s innovative technology on patient care throughout VUMC. The program also underscores both Masimo’s and VUMC’s commitment to leveraging technology to rethink and improve patient care pathways, setting a new standard for how hospitals manage patient surges in the ED and beyond.
“Rising patient acuity and volume at VUMC necessitate strategic initiatives to augment our care infrastructure,” said Neal Patel, MD, MPH, Professor of Clinical Pediatrics and Chief Informatics Officer for HealthIT at VUMC. “Wireless physiologic monitoring in the ED enhances surveillance and vigilance of each patient’s status even when they are in the waiting room.”
Bilal Muhsin, Chief Operating Officer of Masimo, said, “We are excited to partner with Vanderbilt University Medical Center to bring Radius VSM to vulnerable patients in the emergency department, where continuous monitoring is not the norm. A core tenet of our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications—and one of the ways we achieve that is through continued innovation. With Radius VSM, clinicians have the power of cutting-edge technology in a modular, scalable design that’s both easy to use and comfortable for the patient. The applications are virtually limitless, and I cannot wait to see how the use of this technology is expanded to enhance patient safety not only in the emergency department, but across the continuum of care.”
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® Medical Watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius Tº® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and is not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius VSM® and Masimo Patient SafetyNet™, as well as VUMC’s integration of such technologies in the ED and nontraditional care spaces. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius VSM and Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to our prediction that the initial success of VUMC’s pilot program will result in an expanded rollout within the ED and may lead to adoption in other areas of the hospital, broadening the impact of Masimo technology; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
| Last Trade: | US$172.72 |
| Daily Change: | 1.03 0.60 |
| Daily Volume: | 45,535 |
| Market Cap: | US$9.250B |
November 26, 2024 November 05, 2024 September 25, 2024 September 14, 2024 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB