KELOWNA, BC / ACCESSWIRE / March 2, 2023 / Lexaria Bioscience Corp. (Nasdaq:LEXX) (Nasdaq:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces that its diabetes animal model study DIAB-A22-1 has completed and produced at least three positive outcomes including weight loss in obese diabetic-conditioned animals, together with improved triglyceride and cholesterol levels.
REDUCED BODY WEIGHT
Beginning just four days after the start of dosing with DehydraTECH-CBD, the obese rats began to lose weight. The weight loss was maximized nine days after dosing and maintained throughout the 8-week study duration. This apparent trend demonstrated roughly a 7% loss of body weight throughout the course of treatment at both DehydraTECH-CBD doses studied (30 mg/Kg and 100 mg/Kg). Only the DehydraTECH-CBD-dosed animals weighed less at the end of the study than at the beginning, whereas the weight of the untreated obese animals trended upwards throughout the study. The lean untreated animals gained the most weight of all to actually become obese by the end of the study.
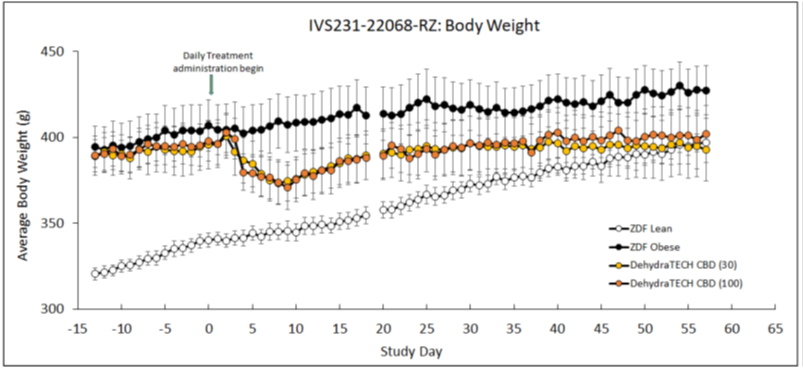
Furthermore, in the DehydraTECH-CBD treated rats the weight loss trend observed did not appear to be a result of lower levels of food or water intake. Food and water intake was comparable in both the treated and untreated obese animal groups suggesting that the weight loss was more so related to enhanced metabolic function.
According to the National Institute for Health, obesity or being overweight is the leading cause of diabetes.
ACTIVITY LEVELS
Obesity is often accompanied by reduced activity levels, which were measured in this study via locomotor activity, the distance the animals travelled in open field observations. Interestingly, the lower dose of DehydraTECH-CBD resulted in a statistically significant improvement in locomotor activity compared to the untreated obese control rats (*p<0.05), whereas there was no significant difference accordingly evidenced at the higher dose. As expected, activity levels for all obese rats were significantly lower than the lean control rats (#p<0.05), although only the lower dose DehydraTECH-CBD treated rats approached the activity levels of the lean rats. Previous research has suggested that CBD may enhance motor activity through its action upon the serotonin 5-HT1A receptor.
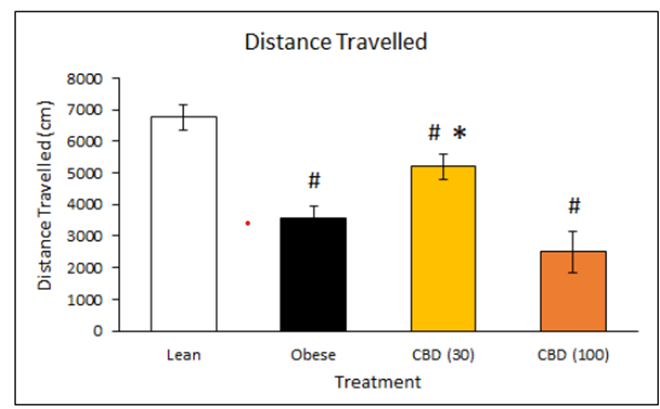
Additional investigation will be required to learn what an optimum DehydraTECH-CBD dose related to both weight loss and increased physical activity might be, given the fact that the higher dose studied may have elicited sedative-like effects of CBD triggering hypolocomotion which others have also observed upon high systemic exposure.
TRIGLYCERIDE AND CHOLESTEROL LEVELS
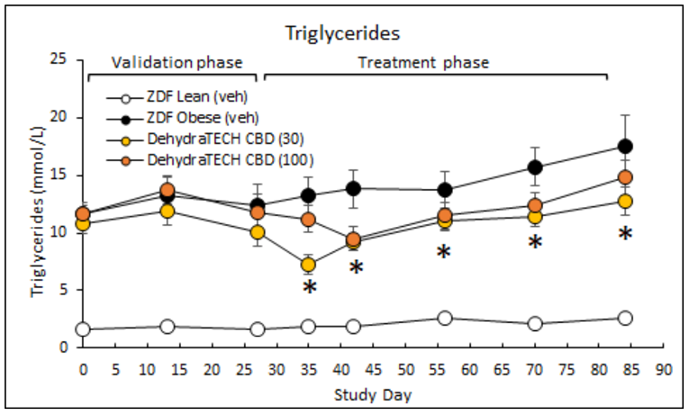
Higher triglyceride levels in the blood can be an indication of the onset of diabetes. One of the best ways to manage triglyceride levels is to lose weight. In study DIAB-A22-1, the animals dosed with DehydraTECH-CBD showed statistically significant reductions in triglyceride levels from day 35 onwards compared to the obese animals not dosed with DehydraTECH-CBD. Once again, the lower dose of 30 mg/Kg of DehydraTECH-CBD outperformed the higher dose of 100mg/kg. By the end of the study the triglyceride levels in the animals receiving 30 mg/Kg of DehydraTECH-CBD was over 25% lower than that of the untreated obese animals (*p=0.007).
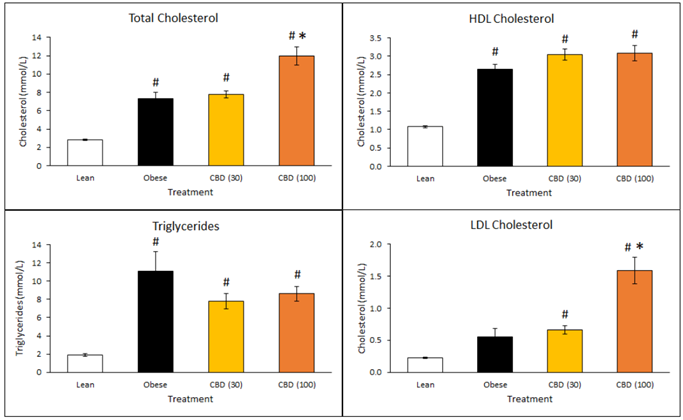
Cholesterol levels in the animals receiving DehydraTECH-CBD were also higher. There are two types of cholesterol - HDL and LDL - where HDL cholesterol is generally considered "good" and is desired and LDL cholesterol is generally considered "bad", although some studies are beginning to question whether this is true. Again, the lower dose of 30 mg/Kg appeared to outperform the higher 100 mg/Kg dose of DehydraTECH-CBD, with a trend toward increased HDL good cholesterol but with no significant effect upon LDL bad cholesterol levels compared to the untreated obese animals. The 100 mg/Kg dosed animals demonstrated increases in both desired HDL and the undesired LDL levels, with significance relative to the untreated obese animals in the latter instance (*p<0.05). The findings with the lower dose therefore appeared to support improved physiological function relative to the diabetic state.
According to the American Diabetes Association, CBD is not thought to directly reduce blood glucose levels as previous clinical studies have discovered, nor was any significant effect from DehydraTECH-CBD dosing on blood glucose measured or expected in this study.
SUMMARY
Animal study DIAB-A22-1 evidenced, at times, dramatic changes in body weight, general activity, and triglyceride and cholesterol levels associated with administration of DehydraTECH-CBD. These findings are well aligned with other study work in animals that points to the known anti-inflammatory and antioxidant properties of CBD functioning to lessen some of the essential pathophysiological factors associated with diabetes. Lexaria is pleased that relatively low doses of DehydraTECH-CBD seem to be supportive of real improvements in day-to-day health of the lab animals and the Company is very encouraged by the positive findings from its first study of DehydraTECH-CBD in this diabetes animal model, indicating many prospective benefits worthy of further investigation together, perhaps, with other drugs that further help to control glucose levels directly.
ABOUT THE STUDY
Study DIAB-A22-1 administered select Lexaria "DehydraTECH-CBD 2.0" formulations at doses of either 30 mg/Kg or 100 mg/Kg of body weight in a total of 32 male Zucker "ZDF" rats - 24 obese and 8 lean. The study length was doubled from four weeks of dosing to eight weeks of dosing, after initial positive early responses were witnessed. Treatment was well tolerated throughout the course of dosing with no serious health concerns observed in the animals that received DehydraTECH-CBD. The health of some of the obese animals declined after the study had concluded as might be expected, likely as a result of the advanced pathology and possible ketoacidosis from the prolonged diabetic condition of these disease-model animals. Study DIAB-A22-1 was undertaken by a leading, third-party testing laboratory located in Canada.
ABOUT THE DIABETES MARKET OPPORTUNITY
Diabetes is a disease whereby the body does not produce sufficient insulin, leading to higher than normal levels of sugars in the blood. Risks of kidney disease, vision loss, heart and cardiovascular disease and more are greatly enhanced by individuals with diabetes. Because diabetes is often closely connected to obesity, it is a chronic and growing problem around much of the world.
People with type-2 diabetes can often control the disease through lifestyle changes and/or taking certain diabetes medications, whereas those with type-1 diabetes are required to add insulin to their body through syringes, insulin pumps, or other similar devices. Thus the treatment of diabetes includes devices, drugs, and lifestyle alteration. The global diabetes devices market is estimated at $26.7 billion in 2021. The global diabetes drug market is estimated at $63.1 billion in 2021. Due to the pain and unpleasantness of injections, many diabetes sufferers prefer to treat their condition with drugs rather than devices, if they have the choice. More than 1.9 billion people were overweight in 2016, and due to the growth in obesity, which has nearly tripled from 1975 to 2016, the number of people experiencing diabetes continues to grow.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 28 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
Phone: 250-765-6424, ext 202
| Last Trade: | US$2.21 |
| Daily Change: | -0.08 -3.49 |
| Daily Volume: | 109,504 |
| Market Cap: | US$38.560M |
December 19, 2024 December 17, 2024 December 09, 2024 November 20, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB