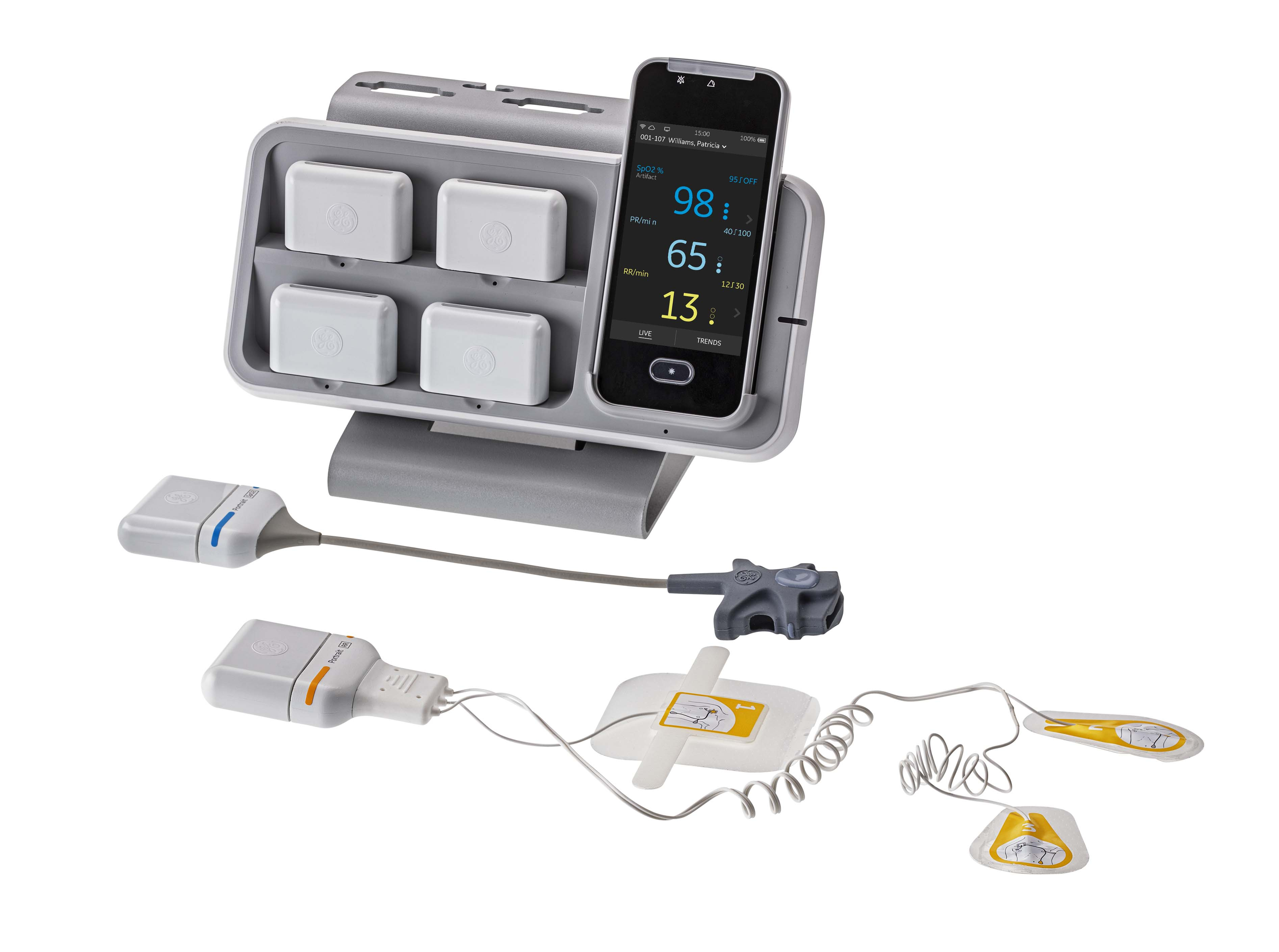
CHICAGO / Aug 14, 2023 / Business Wire / GE HealthCare (Nasdaq: GEHC) today announced it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its Portrait Mobile wireless and wearable monitoring solution. The Portrait Mobile platform enables real-time continuous monitoring with a personalized view of the patient’s vitals while keeping patients mobile during critical recovery periods, especially after surgery or discharge from the intensive care unit.
Continuously monitoring patients and having a real time view of data can help clinicians recognize deterioration earlier than traditional spot check methods, which typically occur only every 4-6 hours. The wireless patient-worn sensors combined with the smart phone-sized monitor eliminate all traditional tethers, allowing patients to move about the ward freely, key to helping improve outcomes and reduce length of stay3.
Portrait Mobile draws on GE HealthCare’s track record in parameter excellence to modernize respiration rate measurement through novel wireless sensor technology. The system’s dual-vector respiration rate measurement technology leverages an innovative algorithm designed for mobile patients, better capturing continuous respiratory rate through optimized electrode placement even with changing breathing patterns.
“Patients recovering from major surgery are fragile. Most patients currently have vital signs monitored every 4-6 hours. We have shown that vital sign abnormalities are common – and sometimes profound and prolonged,” shares Dr. Daniel Sessler, Michael Cudahy Professor and Chair of Outcomes Research at Cleveland Clinic, as well as the Principal Investigator for a joint trial currently evaluating Portrait Mobile. “Many potentially serious episodes of instability are missed with intermittent vital sign assessments. Continuous vital sign monitoring might help clinicians identify patients who are having difficulty so they can provide help quickly.”
Undetected patient deterioration, particularly post-surgery, can lead to hazardous yet preventable consequences, with 30-day mortality after surgery representing the 3rd leading cause of death globally4. The uninterrupted flow of data and continuous measurement of vital signs, such as respiration rate, oxygen saturation and pulse rate, can help healthcare providers detect patient decline as it is happening, enabling timely intervention before a patient deteriorates.
“It’s important for recovery that patients be able to move around freely while their vital signs are being monitored,” said Neal Sandy, general manager, monitoring solutions, GE HealthCare. “Until Portrait Mobile, patient monitoring required that patients be tethered to their beds, limiting mobility. GE HealthCare designed Portrait Mobile with this need in mind – the advent of a small wearable, wireless inpatient monitoring solution that provides reliable monitoring to the patient's care team, while allowing for more patient freedom and flexibility during recovery, is an important advancement in acute care.”
Portrait Mobile is part of GE HealthCare’s FlexAcuity monitoring solutions that combine hardware and software engineered to adapt to rapidly changing patient needs and builds on a well-established history of clinical advancements. It was this deep understanding of patient needs combined with a commitment to providing caregivers with actionable insights that led GE HealthCare’s global engineers to develop this next evolution in patient monitoring technology. GE HealthCare’s technology has been recognized globally for its design, receiving the iF Design Gold Award for Product Design in 2022 for Portrait Mobile and an iF Design Award in 2023 for CARESCAPE Canvas.
For more information on Portrait Mobile, please visit: https://www.gehealthcare.com/products/patient-monitoring/portrait-mobile/
ABOUT GE HEALTHCARE TECHNOLOGIES INC.
GE HealthCare is a leading global medical technology, pharmaceutical diagnostics, and digital solutions innovator, dedicated to providing integrated solutions, services, and data analytics to make hospitals more efficient, clinicians more effective, therapies more precise, and patients healthier and happier. Serving patients and providers for more than 100 years, GE HealthCare is advancing personalized, connected, and compassionate care, while simplifying the patient’s journey across the care pathway. Together our Imaging, Ultrasound, Patient Care Solutions, and Pharmaceutical Diagnostics businesses help improve patient care from prevention and screening, to diagnosis, treatment, therapy, and monitoring. We are an $18 billion business with 50,000 employees working to create a world where healthcare has no limits.
Follow us on Facebook, LinkedIn, Twitter, Instagram and Insights for the latest news, or visit our website www.gehealthcare.com for more information.
1 Loughlin, et al., Respiratory Rate: The Forgotten Vital Sign – Make It Count! Jt Comm J Qual Patient Saf; 2018; 44(8), 494-499.
2 Kelly, C. Respiratory rate 1: why accurate measurement and recording are crucial. Nursing Times 2018; 114: 4, 23-24.
3 Vincent JL, Einav S, Pearse R, Jaber S, Kranke P, Overdyk FJ, Whitaker DK, Gordo F, Dahan A, Hoeft A. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018 May;35(5):325-333. doi: 10.1097/EJA.0000000000000798. PMID: 29474347; PMCID: PMC5902137.
4 The Lancet, The Global Burden of Post-operative Death, https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)33139-8/fulltext#gr1, accessed July 24, 2023.


| Last Trade: | US$77.09 |
| Daily Change: | -2.16 -2.73 |
| Daily Volume: | 3,055,620 |
| Market Cap: | US$35.220B |
December 05, 2024 December 03, 2024 December 01, 2024 | |

Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MORE
Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB