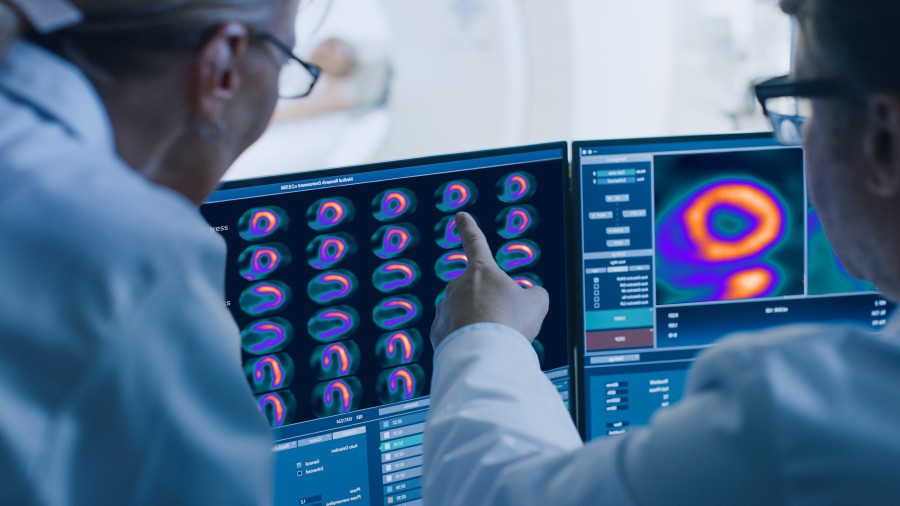
ARLINGTON HEIGHTS, Ill. / Sep 27, 2024 / Business Wire / GE HealthCare (Nasdaq: GEHC) today announced that the U.S. Food and Drug Administration (FDA) has granted approval of Flyrcado™ (flurpiridaz F 18) injection, a first of its kind positron emission tomography myocardial perfusion imaging (PET MPI) agent, for the detection of coronary artery disease (CAD). Indicated for patients with known or suspected CAD, Flyrcado delivers higher diagnostic efficacy compared to single-photon emission computed tomography (SPECT) MPI, the predominant procedure used in nuclear cardiology today. Flyrcado, which can be manufactured in an offsite pharmacy and delivered as a ready-to-use unit dose, has the potential to expand clinician and patient access to PET MPI, including improving diagnostic accuracy in difficult-to-image patients such as those with a high body mass index (BMI) and women1.
With a half-life of 109-minutes—significantly longer than existing PET MPI tracers—Flyrcado removes the need for on-site tracer production and generator maintenance and enables distribution to a wide network of hospitals and imaging centers. This longer half-life also means Flyrcado brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD, enabling the most robust protocol for evaluating ischemia in patients. Furthermore, clinicians would have the ability to rescan a patient during the same imaging session in the event of technical difficulties, rather than rescheduling an additional scan.
Dr. Jamshid Maddahi, MD, FACC, MASNC, principal investigator of the Flyrcado clinical trials, clinical professor of medicine (cardiology) and molecular and medical pharmacology (nuclear medicine) at UCLA School of Medicine and director of the Biomedical Imaging Institute, said, “Flyrcado is the most exciting development in the field of nuclear cardiology over the past few decades. Although PET MPI as a modality enables high diagnostic accuracy as compared to SPECT MPI2, only a minority of annual PET scans in the US are PET MPI because of limited access to the currently available PET tracers—which may be addressed with the introduction of Flyrcado. I am excited for this new radiotracer and its potential impact, as a game changer, for diagnosing the disease with the highest mortality rate in the world.”
CAD is the most common form of heart disease and remains the leading cause of death for men and women in the U.S.3, with 695,000 deaths recorded in 20214. During the multicenter international AURORA Phase III trial, flurpiridaz F 18 was compared with both invasive coronary angiography as a standard of truth to determine diagnostic efficacy in detecting CAD, as well as with SPECT MPI. Around six million MPI procedures are undertaken each year in the U.S.5 to show blood flow through the heart muscle, and evaluate the presence, extent and degree of myocardial ischemia or infarction.
Dr. Mouaz Al-Mallah, MD, MSc, MASNC, immediate Past President of the American Society of Nuclear Cardiology (ASNC) and Director of Cardiac PET at Houston Methodist Hospital, said “Given the desirable properties of flurpiridaz F 18, both in terms of myocardial uptake and fluorine-18 imaging characteristics, Flyrcado represents a favorable combination of imaging agent pharmacology and convenience to imaging institutions and patients. There are new frontiers for cardiac PET that this tracer can achieve; it can be ordered as a unit dose, and it offers the flexibility to perform exercise stress testing. We expect new imaging centers to be able to offer cardiac PET to their patients, making it more convenient to access PET MPI and providing a meaningful impact for clinicians and their patients.”
Kevin O’Neill, CEO of the Pharmaceutical Diagnostics (PDx) segment of GE HealthCare, said, “As the first and only FDA-approved F 18 PET MPI radiotracer for CAD detection, Flyrcado can make a real difference to clinicians and their patients. This is another example of GE HealthCare’s commitment to innovating and investing to shape the future of molecular imaging, increasing diagnostic confidence and addressing unmet patient needs.”
Flyrcado is one of three F 18 imaging agents in GE HealthCare’s portfolio of FDA-approved molecular imaging PET products, joining radiopharmaceutical PET imaging agents Cerianna™ (fluoroestradiol F 18) injection, used to detect estrogen receptor positive lesions as an adjunct to biopsy in patients with metastatic and recurrent breast cancer, and Vizamyl™ (flutemetamol F 18) injection which is a PET tracer for imaging of the brain to estimate beta amyloid neuritic plaque density in adult patients who are being evaluated for Alzheimer’s disease or other causes of cognitive decline.
GE HealthCare acquired exclusive global commercialization rights for flurpiridaz F 18 from Lantheus in 2017 and has led the funding and development of the product through to approval. Lantheus collaborated on the development and will also collaborate on commercialization through a joint steering committee. Lantheus is entitled to royalties based on commercial sales milestones.
Flyrcado will be available in initial U.S. markets in early 2025 with expanding availability thereafter.
As a leading global medical technology and pharmaceutical diagnostics innovator, GE HealthCare provides both molecular imaging equipment and proprietary radiopharmaceuticals used across cardiology, neurology and oncology. The PDx segment is a global leader in imaging agents used to support over 120 million patient procedures per year globally, equivalent to four patient procedures every second.
About GE HealthCare Technologies Inc.
GE HealthCare is a leading global medical technology, pharmaceutical diagnostics, and digital solutions innovator, dedicated to providing integrated solutions, services, and data analytics to make hospitals more efficient, clinicians more effective, therapies more precise, and patients healthier and happier. Serving patients and providers for more than 125 years, GE HealthCare is advancing personalized, connected, and compassionate care, while simplifying the patient’s journey across the care pathway. Together our Imaging, Ultrasound, Patient Care Solutions, and Pharmaceutical Diagnostics businesses help improve patient care from diagnosis, to therapy, to monitoring. We are a $19.6 billion business with approximately 51,000 colleagues working to create a world where healthcare has no limits.
Follow us on LinkedIn, X , Facebook, Instagram, and Insights for the latest news, or visit our website https://www.gehealthcare.com/ for more information.
https://www.gehealthcare.com/products/molecular-imaging-agents/flyrcado
INDICATIONS AND USAGE OF FLYRCADO™:
FLYRCADO™ (flurpiridaz F 18) injection, for intravenous use important safety information
IMPORTANT SAFETY INFORMATION
Indications and Usage
FLYRCADO is a radioactive diagnostic drug indicated for positron emission tomography (PET) myocardial perfusion imaging (MPI) under rest or stress (pharmacologic or exercise) in adult patients with known or suspected coronary artery disease (CAD) to evaluate for myocardial ischemia and infarction.
Contraindications
None
Warnings and Precautions
Adverse Reactions
Use in Specific Populations
There are no data on use of flurpiridaz F 18 in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from flurpiridaz F 18 and the gestational timing of exposure.
FLYRCADO contains ethanol (a maximum daily dose of 337 mg anhydrous ethanol). If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes associated with ethanol exposure during pregnancy.
Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for at least 8 hours after FLYRCADO administration.
Safety and effectiveness of FLYRCADO in pediatric patients have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 800-654-0118 (option 2 then option 1) or by email at This email address is being protected from spambots. You need JavaScript enabled to view it. or FDA at 800-FDA-1088 or www.fda.gov/medwatch
For full prescribing information, click here. For important safety information, please click here.
INDICATIONS AND USAGE OF CERIANNA™:
INDICATIONS AND USAGE
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
CONTRAINDICATIONS
None.
ADVERSE REACTIONS
In Clinical Trials (n=1207) the most common adverse reactions seen occurred at a rate < 1% were injection-site pain and dysgeusia.
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare Company at 800.654.0118 (option 2 then option 1) or by email at This email address is being protected from spambots. You need JavaScript enabled to view it. or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For full Prescribing Information click here.
INDICATIONS AND USAGE OF VIZAMYL™:
PRODUCT INDICATIONS AND USE
VIZAMYL™ (Flutemetamol F 18) injection is indicated for positron-emission tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) or other causes of cognitive decline. A negative scan indicates sparse to no neuritic plaques, inconsistent with a diagnosis of AD at the time of image acquisition. A negative scan result reduces the likelihood that a patient’s cognitive impairment is due to AD. A positive scan indicates moderate to frequent amyloid neuritic plaques. This amount of amyloid neuritic plaque has been shown to be present in patients with AD but may also be present in patients with other neurologic conditions as well as in older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations.
Limitations: A positive scan does not establish a diagnosis of AD or other cognitive disorder. The safety and effectiveness of Vizamyl have not been established for predicting the development of dementia or other neurologic conditions or for monitoring responses to therapies.
Important Safety Information About VIZAMYL™ (Flutemetamol F 18) injection
CONTRAINDICATIONS
• Known hypersensitivity to Vizamyl or any excipient, including polysorbate 80
ADVERSE REACTIONS
• The most commonly reported adverse reactions in clinical trials were flushing (2%), increased blood pressure (2%), headache (1%), nausea and dizziness (1%)
For full prescribing information, including additional important safety information, please click here.
1 Maddahi J, Agostini D, Bateman TM, Bax JJ, Beanlands RSB, BermanDS, Dorbala S, Garcia EV, Feldman J, Heller GV, Knuuti JM, Martinez-Clark P, Pelletier-Galarneau M, Shepple B, Tamaki N, Tranquart F, Udelson JE. Flurpiridaz F-18 PET Myocardial Perfusion Imaging in Patients with Suspected Coronary Artery Disease. J AmColl Cardiol. 2023; 82:1598–1610
2 Schindler, T. H., Bateman, T. M., Berman, D. S., Chareonthaitawee, P., De Blanche, L. E., Dilsizian, V., Dorbala, S., Gropler, R. J., Shaw, L., Soman, P., Winchester, D. E., Verberne, H., Ahuja, S., Beanlands, R. S., Di Carli, M. F., Murthy, V. L., Ruddy, T. D., & Schwartz, R. G. (2020). Appropriate use criteria for PET myocardial perfusion imaging. Journal of Nuclear Medicine, 61(8), 1221-1265. https://doi.org/10.2967/jnumed.120.246280
3 Friede A, O’Carroll PW, Thralls RB, Reid JA. CDC WONDER on the web. Proc AMIA Annu Fall Symp. 1996:408–412.
4 Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation. 2023;147: e93–e621. https://doi.org/10.1161/CIR.0000000000001123.
5 Miller, R. J. H., Bednarski, B. P., Pieszko, K., Kwiecinski, J., Williams, M. C., Shanbhag, A., Liang, J. X., Huang, C., Sharir, T., Hauser, M. T., Dorbala, S., Di Carli, M. F., Fish, M. B., Ruddy, T. D., Bateman, T. M., Einstein, A. J., Kaufmann, P. A., Miller, E. J., Sinusas, A. J., Acampa, W., Han, D., Dey, D., Berman, D. S., & Slomka, P. J. (2024). Clinical phenotypes among patients with normal cardiac perfusion using unsupervised learning: A retrospective observational study. EBioMedicine, 99, 104930. https://doi.org/10.1016/j.ebiom.2023.104930
| Last Trade: | US$77.09 |
| Daily Change: | -2.16 -2.73 |
| Daily Volume: | 3,055,620 |
| Market Cap: | US$35.220B |
December 05, 2024 December 03, 2024 December 01, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB