SOLANA BEACH, Calif., Oct. 31, 2023 (GLOBE NEWSWIRE) -- Evoke Pharma, Inc. (NASDAQ: EVOK), a specialty pharmaceutical company focused primarily on treatments for gastrointestinal (GI) diseases with an emphasis on GIMOTI® (metoclopramide) nasal spray, and EVERSANA, a leading provider of global commercial services to the life science industry, announced a summary of real-world data on the positive impact of GIMOTI (nasal metoclopramide (NMCP) usage in reducing healthcare costs for patients suffering from diabetic gastroparesis (DGP) versus patients taking oral metoclopramide (OMCP). The full abstract entitled “Superiority of Nasal Spray Compared to Orally Administered Metoclopramide in Reducing Healthcare Costs for Treating Diabetic Gastroparesis Patients,” was selected and presented for a plenary session at the American College of Gastroenterology (ACG) 2023 Annual Meeting in Vancouver, Canada. The authors include Dr. David C. Kunkel, Gastroenterologist and Associate Professor of Medicine at UC San Diego Health, Dr. Michael Cline, Gastroenterologist at Cleveland Clinic, and was presented by Dr. Richard McCallum, Gastroenterologist and Professor of Medicine at Texas Tech University, El Paso.
The data describes the results of a comparative study that highlights cost-related healthcare resource utilization data (HCRU) in DGP patients taking GIMOTI (nasal metoclopramide) versus those on oral metoclopramide over a six-month period. HCRU is the description and quantification of patients’ total usage of healthcare services such as emergency department (ED) visits, hospitalization or how often they visit a healthcare provider in the office. These data build on a comprehensive analysis of the HCRU data presented by Dr. David C. Kunkel at Digestive Disease Week (DDW) 2023 earlier this year which demonstrated a significant reduction in healthcare resource usage in patients prescribed GIMOTI compared with patients being prescribed oral metoclopramide. The authors concluded that the likelihood of a DGP patient treated with GIMOTI visiting the ED or being admitted to the hospital was less than half that of patients treated with OMCP during the same period.
DGP is a chronic disorder of the stomach characterized by delayed gastric emptying and a range of symptoms, including nausea, vomiting, early satiety, bloating, and abdominal pain, which drastically reduce a patient’s quality of life. Research shows that patients with diabetic gastroparesis were admitted to the hospital an average of four times per year and that approximately 30% of patients with diabetes admitted for gastroparesis had to be readmitted within 30 days. The cost to the patients and the healthcare system is significant and the impact is considerable for all parties involved. A retrospective U.S. claims study demonstrated that, on average, patients recently diagnosed with diabetic gastroparesis experience higher emergency room costs and greater inpatient and outpatient care costs than diabetic patients without gastroparesis.
For decades, oral metoclopramide has been the most common and only FDA-approved treatment for diabetic gastroparesis. In June 2020, GIMOTI became the first FDA-approved nasal spray for patients suffering from acute and recurrent diabetic gastroparesis, a highly innovative formulation of metoclopramide with a route of administrations that addresses a major limitation in oral therapies which rely on stomach emptying to be absorbed.
With these leading-edge HRCU data in hand, this study aimed to uncover whether reduced healthcare resource utilization would translate to overall health care savings and reductions in costs associated with inpatient hospitalization (IH), emergency department (ED), hospital outpatient (HO), physician office (PO), lab/home/telehealth (LHT), and pharmacy for DGP patients treated with NMCP vs. OMCP.
Using the comprehensive IBM MarketScan Research Database, the data presented at ACG 2023 highlights the actual costs incurred over a 6-month retrospective cohort study by adult patients with documented gastroparesis and diabetes taking either NMCP (n=45) or OMCP (n=180). The cohorts were well matched across age, sex, region, payer, DGP severity, and comorbidity index. Select data points and key findings from the real-world evidence study are outlined below:
Image 1: All-Cause HCRU visits in 6-month post-index period for NMCP and OMCP patients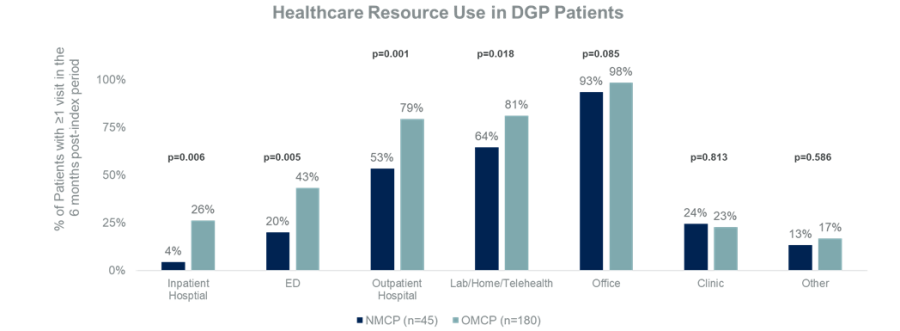
Image 2: All-Cause HRCU Costs between NMCP and OMCP patients over 6-month post index period
Full poster presented at ACG 2023 available on Evoke investor relations website under news and presentations.
“This research provides compelling evidence that route of administration matters and nasal metoclopramide should be used in the preferred position in the treatment of patients with diabetes and gastroparesis. The data for GIMOTI continues to reinforce those providers can and should strive to improve outcomes for patients,” commented Chris Quesenberry, GIMOTI Chief Commercial Officer.
“We continue to witness GIMOTI’s growing market strength amongst all groups of interest, patients and providers primarily. With these cost-benefit data and the recognition by payers (insurers, PBMs, health systems) that there are no good alternatives to reduce the debilitating symptoms and higher utilization of medical benefits by their beneficiaries, we are confident in our ability to improve care for patients with DGP. Patient Access is at the forefront of our mission and with the current economic data available to us, we believe we can expand access to GIMOTI with less financial burden to the healthcare system,” commented David A. Gonyer, R.Ph., Chief Executive Officer of Evoke Pharma.
About Evoke Pharma, Inc.
Evoke is a specialty pharmaceutical company focused primarily on the development of drugs to treat GI disorders and diseases. The company developed, commercialized and markets GIMOTI, a nasal spray formulation of metoclopramide, for the relief of symptoms associated with acute and recurrent diabetic gastroparesis in adults. Diabetic gastroparesis is a GI disorder affecting millions of patients worldwide, in which the stomach takes too long to empty its contents resulting in serious GI symptoms as well as other systemic complications. The gastric delay caused by gastroparesis can compromise absorption of orally administered medications. Prior to FDA approval to commercially market GIMOTI, metoclopramide was only available in oral and injectable formulations and remains the only drug currently approved in the United States to treat gastroparesis.
Visit www.EvokePharma.com for more information.
Follow GIMOTI on Facebook
Follow Evoke Pharma on Facebook
Follow Evoke Pharma on LinkedIn
Follow Evoke Pharma on X
About EVERSANA
EVERSANA® is a leading independent provider of global services to the life sciences industry. The company’s integrated solutions are rooted in the patient experience and span all stages of the product life cycle to deliver long-term, sustainable value for patients, prescribers, channel partners and payers. The company serves more than 650 organizations, including innovative start-ups and established pharmaceutical companies, to advance life sciences solutions for a healthier world. To learn more about EVERSANA, visit eversana.com or connect through LinkedIn and X.
Safe Harbor Statement
Evoke cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negatives of these terms or other similar expressions. These statements are based on the company’s current beliefs and expectations. These forward-looking statements include statements regarding: GIMOTI’s potential to reduce healthcare resource utilization by diabetic gastroparesis patents; and Evoke’s belief that GIMOTI can improve treatment of diabetic gastroparesis. The inclusion of forward-looking statements should not be regarded as a representation by Evoke that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Evoke’s business, including, without limitation: Evoke’s and EVERSANA’s ability to successfully drive market demand for GIMOTI; Evoke’s ability to obtain, maintain and successfully enforce intellectual property protection for GIMOTI; the results of market research studies may not predict acceptance by patients, healthcare providers or payors; inadequate efficacy or unexpected adverse side effects relating to GIMOTI that could result in recalls or product liability claims; Evoke’s ability to obtain additional financing as needed to support its operations; Evoke is entirely dependent on the success of GIMOTI; Evoke’s dependence on third parties for the manufacture of GIMOTI; and other risks and uncertainties detailed in Evoke’s prior press releases and in the periodic reports it files with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Evoke undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
Investor Contact:
Daniel Kontoh-Boateng
DKB Partners
Tel: 862-213-1398
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$5.72 |
| Daily Change: | -0.73 -11.32 |
| Daily Volume: | 64,157 |
| Market Cap: | US$4.690M |
October 28, 2024 October 23, 2024 September 19, 2024 September 04, 2024 August 13, 2024 | |

Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB