BURLINGAME, Calif., June 15, 2023 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (Nasdaq: CRVS), a clinical-stage biopharmaceutical company, today announced new data for CPI-818, the Company’s ITK inhibitor, demonstrating the potential to treat a variety of solid and hematological cancers based on a novel immunotherapy mechanism of action. The data includes updated interim results from the CPI-818 Phase 1/1b clinical trial that continues to demonstrate the potential of ITK inhibition and the absolute lymphocyte count (ALC) biomarker in T cell lymphoma (TCL). The data is being presented in a poster at the International Conference on Malignant Lymphoma (ICML) meeting, which is taking place June 13-17, 2023 in Lugano, Switzerland.
“The CPI-818 data presented at the ICML meeting demonstrates the potential of ITK inhibition to provide a novel immunotherapy mechanism of action for cancer therapy,” said Richard A. Miller, M.D., co-founder, president and chief executive officer of Corvus. “The preclinical and clinical data indicate a consistent and comprehensive rationale based on the biology and mechanism of immune enhancement resulting from selective ITK inhibition. This includes the ability to modulate normal T cell differentiation to enhance and strengthen the immune system’s ability to treat lymphomas and solid tumors. We see this in the interim tumor response data, which showed that a majority of the patients treated with the optimal dose of 200mg twice per day of CPI-818 experience tumor regression. As we consider the landscape of cancer therapy targets, ITK is not in the category of immune checkpoints, but rather it is a kinase that controls whether T cells become cancer killing cells or inflammatory cells. This positioning and the supporting research provide a strong foundation for the continued development of CPI-818 as a monotherapy and its future use in combinations with other therapies. We are excited to build upon these results and plan to meet with the U.S. FDA in the third quarter 2023 to discuss a potential registrational Phase 3 clinical trial for CPI-818 in T cell lymphoma. We also have begun planning for clinical trials with CPI-818 monotherapy in solid tumors. Overall, we have increasing confidence that CPI-818, if approved, can be a novel backbone for various immunotherapies of cancer.”
CPI-818 Data Presented at ICML
The CPI-818 preclinical and clinical data was presented by Ning Ding, Ph.D. from Peking University Cancer Hospital & Institute in Beijing, China, in a poster session (abstract #193) today at the ICML meeting. The poster is available to ICML attendees in the poster hall and e-poster gallery, is also available on the Publications and Presentations page of the Corvus website. The key highlights from the poster presentation include:
Phase 1/1b Clinical Trial Interim Data
CPI-818 is currently being studied in a Phase 1/1b clinical trial as a single agent therapy in patients with relapsed TCL. Corvus recently incorporated a minimum ALC as an eligibility criterion for enrollment in the clinical trial. Based on the current enrollment rate of the Phase 1/1b clinical trial, Corvus believes that the number of patients treated in this clinical trial would provide adequate safety and preliminary efficacy data to inform the design of a potential registrational Phase 3 randomized clinical trial. As recommended by the FDA, Corvus plans to meet with the FDA to discuss such a clinical trial; it is anticipated that this meeting will take place during the third quarter of this year.
Figure 1: Waterfall Plot for Patients in the 200 mg Dose Cohort of the CPI-818 Phase 1/1b Clinical Trial for T Cell Lymphoma. The plot shows the best percent change in tumor volume in the 18 patients (out of 20 total evaluable patients) that were measurable by CT scan. The two other patients had cutaneous and blood involvement; one was a patient with a PR and one patient had progression.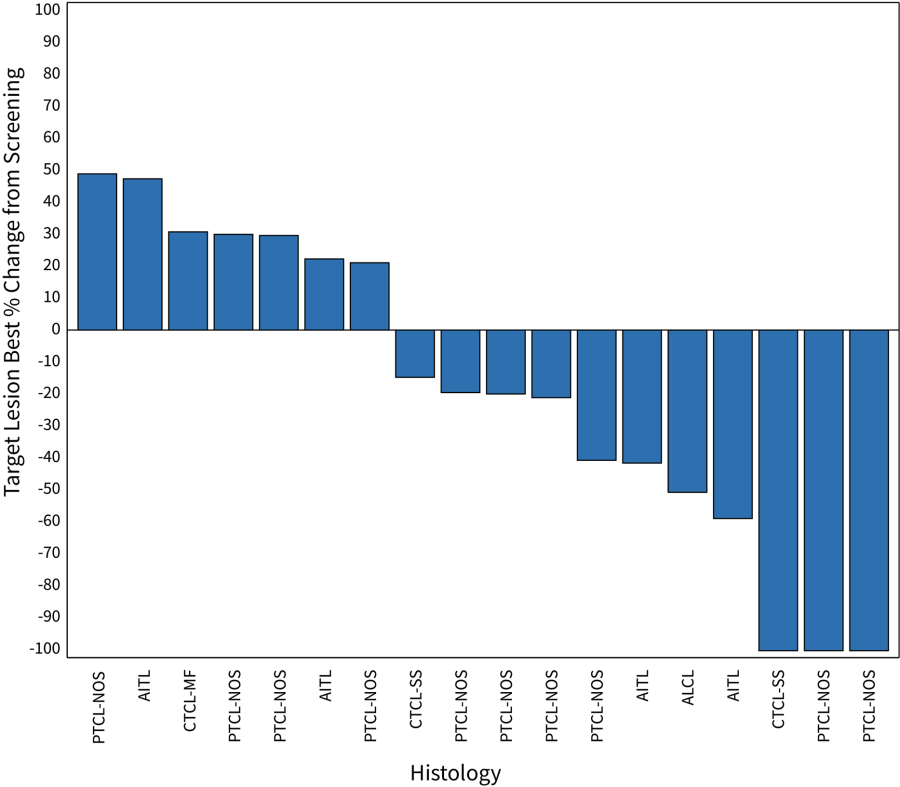
Preclinical Data Supporting CPI-818’s Novel Immunotherapy Mechanism of Action
ITK inhibition with CPI-818 has the potential to treat solid and hematological cancers through a novel mechanism of action that has demonstrated the ability to modulate T cell differentiation and enhance the anti-tumor immune response via Th1 skewing, increased T cell cytolytic capacity and reduction of T cell exhaustion.
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company’s lead product candidate is CPI-818, an investigational, oral, small molecule drug that selectively inhibits ITK and is in a mid-stage clinical trial for patients with T cell lymphoma. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com.
About CPI-818
CPI-818 is an investigational small molecule drug given orally that has selectively inhibited ITK (interleukin-2-inducible T cell kinase) in preclinical studies. ITK, an enzyme, is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. The immunologic effects of CPI-818 lead to what is known as Th1 skewing and is made possible by the high selectivity of CPI-818 for ITK. Recent clinical data in T cell lymphomas, and preclinical studies in murine solid tumor models, suggests that CPI-818 has the potential to control differentiation of normal T helper cells and enhance immune responses to tumors by augmenting the generation of cytotoxic killer T cells and the production of cytokines that inhibit cancer cell survival. Optimal doses of CPI-818 have been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of Th2 related cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases. The Company is conducting a Phase 1/1b trial in patients with refractory T cell lymphomas that was designed to select the optimal dose of CPI-818 and evaluate its safety, PK, target occupancy, immunologic effects, biomarkers and efficacy. Interim data from the Phase 1/1b clinical trial of CPI-818 for T cell lymphoma demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, and identified a dose that maximally drives Th1 skewing.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of the Company’s product candidates including CPI-818; the potential use of CPI-818 to treat a variety of solid tumors and hematological cancers; the Company’s ability and its partners’ ability, as well as the timing thereof, to develop and advance product candidates into and successfully complete preclinical studies and clinical trials, including the Company’s Phase 1/1b clinical trial of CPI-818 and the Company’s planned meeting with the FDA to discuss a registration clinical trial with CPI-818 for T cell lymphoma during the third quarter of this year; the design of clinical trials, including the target number of patients to be enrolled; and the timing of the availability and announcement of clinical data and certain other product development milestones, including whether the Company is able to initiate clinical trials of CPI-818 as a monotherapy for solid tumors. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the three months ended March 31, 2023, filed with the Securities and Exchange Commission on May 8, 2023, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of CPI-818 and its other product candidates; the accuracy of the Company’s estimates relating to its ability to initiate and/or complete preclinical studies and clinical trials; the results of preclinical studies and interim data from clinical trials not being predictive of future results; the unpredictability of the regulatory process; regulatory developments in the United States, and other foreign countries; the costs of clinical trials may exceed expectations; and the Company’s ability to raise additional capital. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
INVESTOR CONTACT:
Leiv Lea
Chief Financial Officer
Corvus Pharmaceuticals, Inc.
+1-650-900-4522
This email address is being protected from spambots. You need JavaScript enabled to view it.
MEDIA CONTACT:
Sheryl Seapy
Real Chemistry
+1-949-903-4750
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$4.70 |
| Daily Change: | -2.69 -36.40 |
| Daily Volume: | 13,989,001 |
| Market Cap: | US$302.020M |
December 18, 2024 December 17, 2024 November 14, 2024 November 12, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB