NEW HAVEN, Conn., June 25, 2024 (GLOBE NEWSWIRE) -- BioXcel Therapeutics, Inc. (Nasdaq: BTAI), a biopharmaceutical company utilizing artificial intelligence to develop transformative medicines in neuroscience and immuno-oncology, today announced positive topline results from a post-marketing requirement (PMR) study of IGALMI™ (dexmedetomidine) sublingual film that demonstrated no evidence of tachyphylaxis, tolerance, or withdrawal with the 180 mcg dose after seven days of PRN (as-needed) treatment.
“We are encouraged by these study results showing a lack of tachyphylaxis, tolerance, and withdrawal at the highest approved dose of IGALMI for patients experiencing frequent episodes of agitation,” said Vincent O’Neill, M.D., Chief of Product Development and Medical Officer of BioXcel Therapeutics. “This new dataset may help address potential changes to the language in the current label. This dataset also adds to our body of evidence for our lead neuroscience asset as we prepare to advance our Phase 3 SERENITY and TRANQUILITY trials.”
PMR Study Methods and Topline Results
In the single-arm, open-label study, 28 inpatient adults with frequent episodes of agitation associated with bipolar disorders or schizophrenia self-administered 180 mcg dose of IGALMI™ as needed over seven days. A total of 83 episodes were treated.
Efficacy Measurements: although this PMR study was not statistically powered to evaluate repeat-dose efficacy, changes in agitation were assessed through the Positive and Negative Syndrome Scale-Excitatory Component (PEC or PANSS-EC) Score and the Clinical Global Impressions – Improvement (CGI-I) Scale, the same measures as used in previous Phase 3 studies.
In addition, no withdrawal or rebound phenomena were observed.
Mean PEC Score Changes During Study Period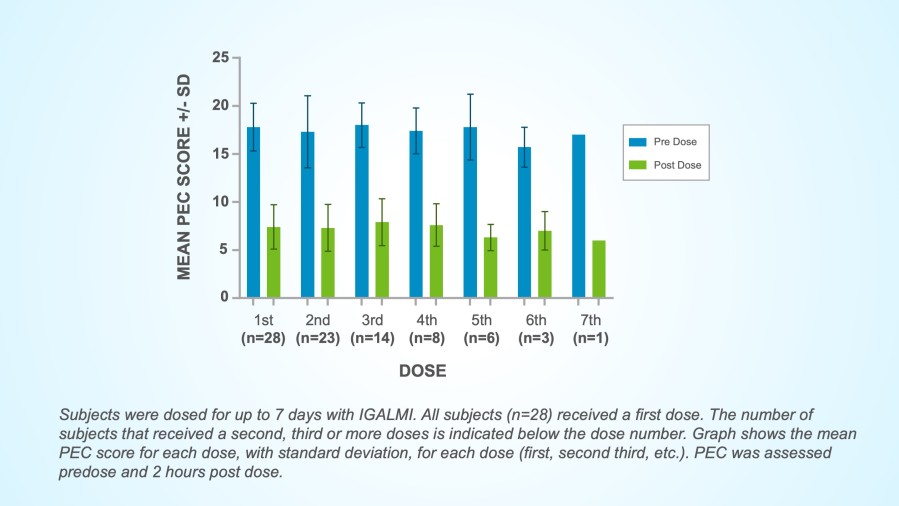
Safety and Tolerability Results: The 180 mcg dose of IGALMI was generally well tolerated and showed favorable safety results in treating patients with frequent episodes of agitation.
“We are pleased these study findings showed consistent responses to PRN treatment for episodes of agitation over the study duration with no evidence of worsening or withdrawal,” said Rob Risinger, M.D., Chief Medical Officer of Neuroscience. “We look forward to sharing these IGALMI PMR data with FDA.”
About IGALMI™ (dexmedetomidine) sublingual film
INDICATION
IGALMI™ (dexmedetomidine) sublingual film is a prescription medicine, administered under the supervision of a health care provider, that is placed under the tongue or behind the lower lip and is used for the acute treatment of agitation associated with schizophrenia and bipolar disorder I or II in adults. The safety and effectiveness of IGALMI has not been studied beyond 24 hours from the first dose. It is not known if IGALMI is safe and effective in children.
IMPORTANT SAFETY INFORMATION
IGALMI can cause serious side effects, including:
The most common side effects of IGALMI in clinical studies were sleepiness or drowsiness, a prickling or tingling sensation or numbness of the mouth, dizziness, dry mouth, low blood pressure, and low blood pressure upon standing.
These are not all the possible side effects of IGALMI. Patients should speak with their healthcare provider for medical advice about side effects.
Patients should tell their healthcare provider about their medical history, including if they suffer from any known heart problems, low potassium, low magnesium, low blood pressure, low heart rate, diabetes, high blood pressure, history of fainting, or liver impairment. They should also tell their healthcare provider if they are pregnant or breastfeeding or take any medicines, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Patients should especially tell their healthcare provider if they take any drugs that lower blood pressure, change heart rate, or take anesthetics, sedatives, hypnotics, and opioids.
Everyone is encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You can also contact BioXcel Therapeutics, Inc. at 1-833-201- 1088 or This email address is being protected from spambots. You need JavaScript enabled to view it..
Please see full Prescribing Information.
About the Positive and Negative Syndrome Scale-Excitatory Component Score (PEC or PANSS-EC)
The PEC total score is a validated endpoint for use in clinical research to quantify the severity of a patient’s acute agitation. The PEC rating evaluates 5 elements associated with agitation: poor impulse control, tension, hostility, uncooperativeness, and excitement; each scored 1 (minimum) to 7 (maximum). The PEC total score is the sum of these 5 elements and thus ranges from 5 to 35.
About the Clinical Global Impressions – Improvement Scale (CGI-I)
The CGI-I scale is a widely used rating scale to assess overall improvement or change in a patient's condition. It provides a subjective evaluation of the patient's global improvement relative to their baseline or previous state. The scale consists of categories ranging from "Very much improved" to "Very much worse," allowing healthcare professionals or researchers to rate the patient's progress based on their clinical judgment.
About BioXcel Therapeutics, Inc.
BioXcel Therapeutics, Inc. (Nasdaq: BTAI) is a biopharmaceutical company utilizing artificial intelligence to develop transformative medicines in neuroscience. Its wholly owned subsidiary, OnkosXcel Therapeutics, is focused on the development of medicines in immuno-oncology. The Company’s drug re-innovation approach leverages existing approved drugs and/or clinically validated product candidates together with big data and proprietary machine learning algorithms to identify new therapeutic indications. For more information, please visit bioxceltherapeutics.com.
Forward-Looking Statements
This press release includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. All statements contained in this press release other than statements of historical fact should be considered forward-looking statements, including, without limitation, statements related to the potential efficacy, safety and tolerability of IGALMI™ (dexmedetomidine) for PRN treatment of agitation associated with bipolar disorders or schizophrenia; the Company’s expectations regarding the warnings and precautions language in the current IGALMITM label; and the potential for the results from the Company’s completed, ongoing and proposed clinical trials to support regulatory approvals for its product candidates; . When used herein, words including “anticipate,” “believe,” “can,” “continue,” “could,” “designed,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements use these words or expressions. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon the Company’s current expectations and various assumptions. The Company believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. The Company may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various important factors, including, without limitation: its limited operating history; its incurrence of significant losses; its need for substantial additional funding and ability to raise capital when needed; the impact of the reprioritization; its significant indebtedness, ability to comply with covenant obligations and potential payment obligations related to such indebtedness and other contractual obligations; the Company has identified conditions and events that raise substantial doubt about its ability to continue as a going concern; its limited experience in drug discovery and drug development; risks related to the TRANQUILITY program; its dependence on the success and commercialization of IGALMI™, BXCL501, BXCL502, BXCL701 and BXCL702 and other product candidates; its lack of experience in marketing and selling drug products; the risk that IGALMI or the Company’s product candidates may not be accepted by physicians or the medical community in general; the Company still faces extensive and ongoing regulatory requirements and obligations for IGALMI; the failure of preliminary data from its clinical studies to predict final study results; failure of its early clinical studies or preclinical studies to predict future clinical studies; its ability to receive regulatory approval for its product candidates; its ability to enroll patients in its clinical trials; undesirable side effects caused by the Company’s product candidates; its novel approach to the discovery and development of product candidates based on EvolverAI; the significant influence of and dependence on BioXcel LLC; its exposure to patent infringement lawsuits; its reliance on third parties; its ability to comply with the extensive regulations applicable to it; impacts from data breaches or cyber-attacks, if any; risks associated with the increased scrutiny relating to environmental, social and governance (ESG) matters; risks associated with federal, state or foreign health care “fraud and abuse” laws; and its ability to commercialize its product candidates, as well as the important factors discussed under the caption “Risk Factors” in its Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2024, as such factors may be updated from time to time in its other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors section of the Company’s website at www.bioxceltherapeutics.com.. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While the Company may elect to update such forward-looking statements at some point in the future, except as required by law, it disclaims any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this press release.
Contact Information
Corporate
BioXcel Therapeutics
Erik Kopp
1.203.494.7062
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media
Russo Partners
David Schull
1.858.717.2310
This email address is being protected from spambots. You need JavaScript enabled to view it.
Scott Stachowiak
1.646.942.5630
This email address is being protected from spambots. You need JavaScript enabled to view it.
IGALMI™ is a trademark of BioXcel Therapeutics, Inc.
BT BIOXCEL THERAPEUTICS is a registered trademark of BioXcel Therapeutics, Inc.
All other trademarks are the properties of their respective owners.
Copyright © 2024, BioXcel Therapeutics, Inc. All rights reserved.

| Last Trade: | US$0.51 |
| Daily Change: | -0.06 -10.80 |
| Daily Volume: | 1,911,212 |
| Market Cap: | US$20.740M |
November 21, 2024 November 14, 2024 October 29, 2024 September 19, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB