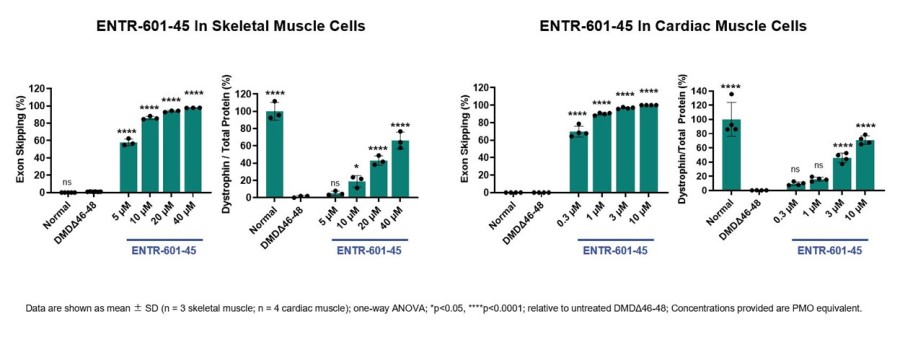
Cash runway extended through the second quarter of 2026 $352 million in cash, cash equivalents and marketable securities as of December 31, 2023 Completed dosing for third cohort of Phase 1 clinical trial of ENTR-601-44 for the potential treatment of DMD with data readout on track for the second half of 2024 Regulatory applications expected in the fourth quarter of 2024 for the global Phase 2 clinical development of... Read More